Risk factors for preeclampsia and eclampsia in Sidama region, southern Ethiopia: a nested case-control study
Birhanu Jikamo, Mulat Adefris, Telake Azale, Kassahun Alemu
Corresponding author: Birhanu Jikamo, Hawassa University College of Medicine and Health Sciences, Hawassa, Southern Nations, Ethiopia 
Received: 22 Mar 2022 - Accepted: 06 Sep 2022 - Published: 09 Sep 2022
Domain: Gynecology, Obstetrics and gynecology, Maternal and child health
Keywords: Pre-eclampsia, eclampsia, risk factors, pregnant women, Ethiopia
©Birhanu Jikamo et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Birhanu Jikamo et al. Risk factors for preeclampsia and eclampsia in Sidama region, southern Ethiopia: a nested case-control study. PAMJ-One Health. 2022;9:3. [doi: 10.11604/pamj-oh.2022.9.3.34409]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/9/3/full
Research 
Risk factors for preeclampsia and eclampsia in Sidama region, southern Ethiopia: a nested case-control study
Risk factors for preeclampsia and eclampsia in Sidama region, southern Ethiopia: a nested case-control study
![]() Birhanu Jikamo1,&, Mulat Adefris2, Telake Azale2,
Birhanu Jikamo1,&, Mulat Adefris2, Telake Azale2, ![]() Kassahun Alemu Gelaye2
Kassahun Alemu Gelaye2
&Corresponding author
Introduction: though Ethiopia has made a significant improvement in the reduction of maternal mortality, the high burden of preeclampsia remains a concern in the Sidama region of southern Ethiopia. This study aimed to determine the risk factors for preeclampsia and eclampsia in the Sidama region of southern Ethiopia.
Methods: a nested case-control study was conducted from August 8, 2019, to October 1, 2020 in the Sidama region. Two-stage sampling techniques were used to recruit study participants. First, seven of the 13 public hospitals were selected using a random sampling technique. Second, cases and controls were selected from a cohort of pregnant women enrolled at ≥20 weeks of gestation up until the 37th week. Data were collected in a face-to-face interview using a locally translated and validated tool. Binary logistic regression analysis was used to identify risk factors for preeclampsia and eclampsia.
Results: of the planned sample size of 816 women, we enrolled 808 (404 cases and 404 controls). Of the 404 cases, (59.40%, 240/404) had preeclampsia without severity features, (30.94%, 125/404) had preeclampsia with severity features, and (9.65%, 39/404) had convulsions. After controlling for confounders, women having a low wealth status were 98% [AOR: 1.98, 95%CI: 1.34-2.92] at higher risk for preeclampsia and eclampsia compared to women having a high wealth status. Women who had early neonatal deaths were 5 times more likely to be developed preeclampsia and eclampsia than women who did not have early neonatal deaths [AOR: 5.09, 95%CI: 1.69-9.36]. Women who did not attend school were three times more likely to develop preeclampsia and eclampsia [AOR: 3.00, 95% CI: 1.10-8.19] compared to women who attended college/university.
Conclusion: in this study, a higher risk for preeclampsia and eclampsia was observed among women with low wealth status, women who had early neonatal deaths and women who did not attend school. Some of these factors could be positively influenced by educational interventions. Maternal and child health providers should screen pregnant women at risk for preeclampsia and eclampsia using these factors. Findings of this study will provide epidemiological evidence for policy makers and implementers to reduce the occurrence of preeclampsia and eclampsia.
Preeclampsia and eclampsia are two of the most common hypertensive disorders of pregnancy (HDPs) [1]. It is the second leading cause of direct maternal death and is directly responsible for 70,000 maternal deaths annually at the global level [2]. In low-and middle-income countries (LMICs), 10-15% of direct maternal mortalities were associated with preeclampsia and eclampsia in 2018. In Ethiopia in 2019, the pooled prevalence of maternal death was 4% [3]. In Ethiopia, in 2018, the overall pooled prevalence of HDPs was 6.07% [4]. In the same study, a higher pooled prevalence of HDPs was observed in southern Ethiopia (10.13%), with the lowest prevalence observed in Addis Ababa, the capital city of Ethiopia (5.41%) [4]. Moreover, it is associated with an increased risk of adverse neonatal outcomes, including preterm birth, fetal growth restriction, and intrauterine fetal death. Although maternal outcomes as hepatic failure, antepartum and postpartum hemorrhage, and stroke [5]. The most vulnerable populations for pregnancy, delivery and postpartum-related complications and deaths are pregnant women in LMICs [6]. This is because they face challenges linked to lower education levels, health literacy, and limited access to and use of antenatal care services [7]. In sub-Saharan Africa (SSA), 68% of women report at least one antenatal care visit [7].
In SSA, hypertensive disorders of pregnancy (HDPs) are the second leading cause of maternal mortality (22.1%), followed by obstetric hemorrhage (28.8%) in 2020 [8]. In 2019, HDPs are the third leading cause of maternal deaths in southern Ethiopia (16%), followed by obstetric hemorrhage (39%), and anemia (28%) [9]. The main pregnancy complications for admission to a hospital in 2019 in southern Ethiopia were severe preeclampsia (51.8%), followed by postpartum hemorrhage (24.9%) [9]. Furthermore, eclampsia accounted for 70% of pregnancy complications that occurred after 12 hours of admission [9]. A number of factors account for the occurrence of preeclampsia and/or eclampsia, including maternal age, family history of hypertension or preeclampsia, preeclampsia in the past pregnancys, lack of prenatal care, body mass index ≥30, diabetes mellitus, chronic hypertension, history of preterm birth or stillbirth, parity, drinking alcohol, history of induced abortion and multiple pregnancies [10-15]. Women who visited antenatal care in the first trimester were at higher odds to have good knowledge of preeclampsia compared to those women who visited ANC in the third trimester [16]. Women who gave birth at the health institution were 2.61 times higher odds to have good knowledge of preeclampsia compared to those women who gave birth at the home [16]. In the Sidama region, 38% of women were aware of being hypertensive before the study [17]. In addition, in 2020, 22.7% of pregnant women did not know the causes of newborn death in Sidama region [18]. The third Sustainable Development Goal (SDGs) is planned to reduce the global maternal mortality ratio to less than 70 per 100,000 live births by 2030 [19]. As a result, Ethiopia's government has set a goal of lowering maternal deaths from 401 to 140 per 100,000 live births by 2030 [20]. However, the high burden of preeclampsia is a concern in the Sidama region of Ethiopia, even though the country has made a significant improvement in the reduction of maternal mortality up to 401 per 100,000 live births. This suggests a need for understanding risk factors for preeclampsia and eclampsia [20].
Previous studies in southern Ethiopia revealed inconsistencies in reporting factors associated with preeclampsia and eclampsia [15,21-23]. A result did not show the association between preeclampsia/eclampsia and its risk factors [23]. Another study in Ethiopia did not include a non-exposed group that would have been important to controlling confounders like the quality of maternal care associated with preeclampsia and eclampsia [24]. What is more, the studies were poor in generating evidence that could be used by policymakers and in clinical practices, did not include control groups, and didn´t measure the risk of outcomes of interest. Results of this study could be shared with maternal and child health providers in the region so that these risks are detected and controlled early in pregnancy. In addition, finding of this study will be used for providing care and treatment for women who experienced preeclampsia/eclampsia. What are the risk factors for preeclampsia and eclampsia among pregnant women attending antenatal care in the Sidama region? Therefore, this study aimed to determine the risk factors for preeclampsia and eclampsia in the Sidama region of southern Ethiopia.
Study design: this was a nested case-control study conducted from August 8, 2019, to October 1, 2020 in the Sidama region.
Setting: in 2020, the population of the region was approximately 4 million. There were thirteen public hospitals, 138 health centers, and 540 health posts in the region that provided maternal, newborn, and child health services. In 2020, approximately 132,031 pregnant women attended ≥4 antenatal care visits (ANC) and 127,585 births were assisted by skilled birth attendants. Out of the 13 hospitals that are found in the region, we enrolled participants from seven of the hospitals, including Adare, Hawassa, Yirgalem, Hula, Bona, Chuko, and Daye hospitals.
Participants
Eligibility criterion
Inclusion criteria: pregnant women with hypertension plus proteinuria, mild hypertension and evidence of organ dysfunction, severe hypertensive without proteinuria and evidence of organ dysfunction were included in the study [25,26].
Cases: cases (pre-eclampsia and eclampsia) were diagnosed in accordance with the obstetrics management protocol for Hospitals in Ethiopia in 2021, as well as the most recent International Society for the Study of Hypertension in Pregnancy guidelines [25,26]. Preeclampsia was diagnosed with the minimum criteria of the presence of proteinuria (≥1+ or 0.3 g/L) and hypertension (≥140/90 mmHg) on two occasions at least 4 hours apart, detected after the 20th week of gestation up until the 37th week of pregnancy in a previously normotensive woman. Alternatively, we diagnosed high blood pressure combined with multi-systemic manifestations such as HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, renal in-sufficiency, pulmonary edema, and visual or cerebral disturbances that supported the diagnosis of PE even in the absence of proteinuria. Cases of a disease (pre-eclampsia and eclampsia) that occurred in a defined cohort of pregnant women after the 20th week of gestation up until the 37th week of pregnancy in a previously normotensive woman. Eclampsia was diagnosed as the presence of convulsions that could not be attributed to other causes in a woman with preeclampsia.
Controls: controls were diagnosed as women with a blood pressure of < 140/90 mmHg and no confirmed proteinuria detected after the 20th week of gestation up until the 37th week of pregnancy or no history of preeclampsia and eclampsia were recruited as controls. Cases and controls were selected from a cohort of pregnant women enrolled at ≥20 weeks of gestation up until the 37th week of pregnancy. For each case, a single control was recruited from among those in the cohort of pregnant women who had not developed the disease (no history of preeclampsia and eclampsia) by the same time of the disease occurrence in the case. Cases and controls were selected after the physicians´ made diagnoses for women who developed preeclampsia and eclampsia and controls, including general medical practitioners, emergency surgical officers, or obstetricians/gynecologists during the antenatal care follow-up.
Operational definitions: preeclampsia without severe features were defined as one or more of the following conditions: absence of systemic involvement and raised BP ≥140/90 mmHg plus 24-hour urine protein ≥300mg/24 hour or urine dipstick >+1 after 20 weeks of gestation in previously normotensive women. Preeclampsia with severe features was defined as one or more of the following conditions: BP≥160/110 mmHg, hepatic dysfunction, pulmonary edema and/altered mental status, headache, blurred vision, right upper quadrant pain, blindness, seizures and disseminated intravascular coagulation, and elevated liver enzymes [26]. Gestational age was calculated based on a woman´s recall of her last menstrual period. However, an ultrasound scan was used for those women who could not remember their last menstrual period [26].
Study variables
Dependent variable: the primary outcome of interest for this study was preeclampsia and eclampsia. They were combined into a single outcome variable as pre-eclampsia and eclampsia [27]. The outcome variable was identified based on having the recorded diagnosis (1 for those women with the outcome and 0 for those women without the outcome).
Independent variables: we included study variables based on the research question and an extensive review of the preeclampsia and eclampsia literature. The following risk factors for preeclampsia and eclampsia were identified. Socio-demographic and economic variables were collected including: maternal age (16-24, 25-34 and >35 years), religion (orthodox, protestant, Muslim and others (catholic and Jova), residence (urban and rural), maternal education (no formal education, primary education, secondary education and college/university, husband's education (no formal education, primary education, secondary education and college/university), maternal occupation (housewife, merchant, employed, farmer, daily laborer, and student) and husband's occupation (unemployed, merchant, employed, farmer and daily laborer), wealth index classified using principal component analysis as low, middle, and high. Maternal and fetal characteristics variables were collected in each hospital through medical records and using a checklist including: fetal sex (male and female), number of neonates delivered (singleton and twins), stillbirth (yes/no), gestational age at admission (<34 and 34-37 weeks), gestational age at delivery (extremely preterm(<28 weeks), very preterm (28-32 weeks, moderate to late preterm (32-37 weeks and term > 37 weeks), parity (nulliparous, 1, 2-3, and >4), gravidity (primigravida, 2-3 and >4), history of cesarean delivery (yes/no and not applicable), ANC in the past pregnancy (yes, no and not applicable), history of 3 or more consecutive spontaneous abortion (yes/ no and not applicable), birth weight of last baby<2500g (yes/no, and not applicable), birth weight of last baby >4000g (yes/no, and not applicable), family history of hypertension (yes/no), maternal history of hypertension (yes/no), history of still birth (yes/no) and maternal history of diabetes (yes/no).
Data collection: we validated the data collection tool before data collection [28]. Two bilingual translators (speakers of both Sidamic and English languages), who were capable of translating the original tool in the English version into the Sidamic version, were selected. Translations into the Sidamic language more accurately reflected the tones of the language. The translations were compared and discrepancies were noted during the translation process. The poorer wording choices were identified and resolved in a discussion between the translators. The back translations were done by two experts of the source language (English). This was a validity checking process to ensure that the translated version reflected the same item content as the original version did. Face and content validation of the tool was done by a panel of experts (midwife experts, epidemiologists, and gynecologists). The panel of experts independently assessed the tool for readability, intelligibility, clarity, and ease of use. The internal consistency for each dimension was checked using Cronbach´s alpha (Cronbach´s alpha=0.98) [28]. In the first pilot test, conducted in a non-study area, all participants responded to all items in the data collection tool and marked them correctly. No missing items were found. Data collectors also reported no difficulty in asking the questions, and no participant reported having any problem understanding the items. The tool was tested for the second time two weeks after the first measurement. The two-week test-retest reliability result was shown to have a good correlation with reliable strategies to assess these point scores (Intraclass Correlation Coefficients (ICC) for agreement 0.78; p< 0.001) because the ICC value was found to be in the range of 0.75 to 0.9, indicating good reliability. We also specified the kind of ICC was calculated, we used the two-way mixed-effects model for calculating ICC as the model of choice for test-retest reliability measure [28]. Trained midwives conducted face-to-face interviews at antenatal care clinics using the pre-tested validated tool. A checklist was used to collect information from the maternal and neonatal records of women with preeclampsia and normotensive women in each hospital. The data collection procedures were supervised by three Maternal and Child Health maternity and reproductive health professionals.
Sample size and sampling: the sample size was calculated using EPI INFO version 7. We considered the following assumptions for sample size calculations: history of stillbirth [29], the ratio of the case to control group (1 to 1), the odds' ratio of 3.13, and the proportion of history of stillbirth among controls was 2.3%. The sample size was estimated to be 816 (408 cases and 408 controls), accounting for a design effect of two and a 10% loss to follow-up. We calculated a two-sided confidence level of 95%, with a power of 80%. Two-stage sampling techniques were used to recruit study participants. First, seven of the 13 public hospitals were selected using a random sampling technique. Second, cases and controls were selected from a cohort of pregnant women enrolled at ≥20 weeks of gestation up until the 37th week.
Statistical analysis: data were cleaned, coded and analyzed using Stata 14. We identified outliers and missing values and checked data consistency using the original questionnaire for the responses using participants´ code numbers. Mean and standard deviations were computed for continuous variables. Frequencies and percentages were computed for categorical variables. Cross tabulation was also performed to test the relationship of exposure variables with the outcome variable. A chi-squared test was used to compare categorical variables between women with cases and controls. Principal component analysis was computed and used for wealth index computation and was ranked in three groups as low, middle, and high. It was a composite measure of household cumulative living standard, and calculated by using data on household ownership of selected assets, like various household assets and means of transportation. Different items for urban and rural areas were computed separately. We included 21 items for rural residents and 16 items for urban residents. The suitability of data was computed by using Bartlett´s test of sphericity and the Kaiser-Meyer-Olkin (KMO) measure of sample adequacy [30]. The KMO >0.6 was used to confirm the sample adequacy for factor analysis [30]. A multivariable binary logistic regression model was performed to identify the risk factors for preeclampsia and eclampsia. According to Hosmer and Lemeshow, a variable with a P-value <0.25 was recommended as a screening criterion for the selection of candidate variables used in a multivariable binary logistic regression model [31]. This confirmed that insignificant variables from the first step were reanalyzed in later steps. Moreover, the candidate variables were also considered based on subject matter expertise, such as gynecologists, obstetricians, epidemiologists, and statisticians who were working as a supervisor, and who provided more subject matter expertise to improve the modeling process substantially. This insight from subject-matter experts substantially improved the modeling process. Adjusted odds' ratio with their 95% confidence interval was reported. Risk factors were assessed comparatively between women with preeclampsia and normotensive women. A variable with <0.05 was used to identify statistically significant risk factors for preeclampsia and eclampsia.
Ethical considerations: this study was reviewed and ethical approval was issued by the Institutional Review Board of the University of Gondar with R.No. O/V/P/RCS/044/2019 in March 2019. All participants signed an informed consent document before study participation began. Pregnant women having abnormal clinical and laboratory results were referred for treatment. Women with severe hypertension were provided with antihypertensive drugs; those with convulsions were given magnesium sulphate.
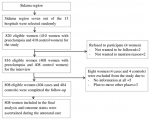
Socio-demographic characteristics of study participants: of the planned sample size of 816 cases and controls, we enrolled 808 (404 cases and 404 controls) participants. Eight (0.98%) of the participants were lost to follow-up. Of these eight participants, four were from the case group and four from the control group. Four participants refused to participate in the study. During the follow-up, eight controls developed preeclampsia. We thus included these eight women in the case group (Figure 1). The mean age of cases was 25.41 ± 4.75 years and that of controls was 24.56 ± 4.58 years. Of the 404 cases, (59.40%, 240/404) had preeclampsia without severity features, (30.94%, 125/404) had preeclampsia with severity features, and (9.65%, 39/404) had convulsions. More than Half of the women with preeclampsia (54%, 218/404) were 16-24 years old in the case group compared to the control group (45%, 182/404, P<0.05). Nearly half of the women who had preeclampsia (44.3%, 179/404) had attended primary school education in the case group, compared to the control group (38.4%, 155/404, P<0.001). During the follow-up, 120 cases and 90 control women were selected at <34 weeks of gestation, and 284 cases and 314 controls were selected at 34-37 weeks of gestation (Table 1).
Maternal and fetal characteristics of women with preeclampsia and eclampsia: compared to the control group (6.2%, 25/404), a higher proportion of women with preeclampsia was observed among women who had a maternal history of hypertension (35.4%, 143/404), P<0.05). A higher proportion of women with preeclampsia (9.4%, 38/404) was reported among women who had a family history of hypertension compared to the control group (6.2%, 25/404, P<0.05). A higher proportion of women with preeclampsia (15.3%, 62/404) was observed among women who did not attend antenatal care in the past pregnancy compared to the control group (14.1%, 57/404, P<0.05) (Table 2).
Risk factors for preeclampsia and eclampsia: in the bivariable logistic regression model, the following variables were identified as candidate variables for multivariable logistic regression analysis: wealth index, maternal education, husband education, fetal sex, maternal age, maternal occupation, family size, place of residence, gravidity, antenatal care follow-up in the past pregnancy, and early neonatal death. After controlling for confounders, we identified significant risk factors for preeclampsia and eclampsia as women 16-24 years old were at 63% [AOR: 1.63, 95%CI: 1.15-2.30] higher risk for preeclampsia and eclampsia compared to women ≥35 years old. Women between the ages of 25 and 34 were seven times more likely [AOR: 7.06, 95% CI: 1.48-13.51] to develop preeclampsia and eclampsia compared to women over the age of 35. Women who did not attend school were three times more likely to develop preeclampsia and eclampsia [AOR: 3.00, 95% CI: 1.10-8.19] compared to women who attended college/university. Women who had early neonatal deaths were 5 times [AOR: 5.09, 95%CI: 1.69-9.36] at higher risk for preeclampsia and eclampsia compared to women who did not have early neonatal deaths. Women with a low wealth status were 98% [AOR: 1.98, 95% CI: 1.34-2.92] more likely to develop preeclampsia and eclampsia compared to women with a high wealth status. Housewife women had a 60% [AOR: 1.60, 95%CI: 1.02-2.50] at higher odds of preeclampsia and eclampsia compared to employed women. Women who resided in rural areas had a 75% [AOR: 1.75, 95% CI: 1.02-3.01] higher risk of preeclampsia and eclampsia compared to women who resided in urban areas (Table 3).
In this study, we identified significant risk factors for preeclampsia and eclampsia as women with low wealth status, those living in rural areas, younger women, women who had early neonatal death, women who did not attend antenatal care visits in the past pregnancy, women who did not attend formal education, and housewife women. Women with low wealth status were at higher risk for preeclampsia and eclampsia compared to women with high wealth status. This finding was similar to findings of another study conducted in Ethiopia, which found that the wealthiest women were two times more likely to have optimal ANC visits compared to women in the poorest wealth status, which creates a conducive environment for early screening of preeclampsia [32,33]. What is more, it could also be due to women with low socioeconomic status having poor health-seeking behavior because they could not afford to pay hospital costs for the services, contributing to poor utilization of antenatal services. This finding was similar to findings of another study conducted in Ethiopia, which found that the wealthiest women were two times more likely to have optimal ANC visits compared to women in the poorest wealth status, which creates a conducive environment for early screening of preeclampsia [32]. Women who resided in rural areas were at higher risk for preeclampsia and eclampsia compared to urban residents. This finding is similar to another study conducted in southern Ethiopia in 2021 that found women who resided in rural areas were at a higher risk of developing pregnancy-induced hypertension compared to women who resided in urban areas [33]. Another study conducted in Tanzania found that women who lived in urban areas had two times the odds of developing HDPs as those who lived in rural areas [34]. This finding was supported by another study conducted in Ethiopia in 2022, which found that rural women have a 42% lower chance of receiving ANC care compared to women who resided in urban areas [32]. This might be due to women who lived in rural areas having a lower socioeconomic status compared to urban residents, which could lead to less health-seeking behavior.
Younger women were at higher risk for preeclampsia and eclampsia compared to older women. This finding was contradicted by another study conducted in Tanzania which found that mothers whose age group was >35 years old had 2.6 times more likely to develop preeclampsia compared to younger women [34]. This finding was consistent with another study conducted in Kenya that showed that women whose age group of 20-34 years old had 1.5 times higher odds of preeclampsia and/or eclampsia, whereas women in the age group of 35-49 years had 2.5 times higher odds of preeclampsia and/or eclampsia compared to women whose age group was < 20 years old [35]. The discrepancy could be due to differences in marriage culture, belief, and religious teachings on marriage, which vary from one country to another, and age categorization among studies varied. Women who did not attend antenatal care in the past pregnancy had a higher risk of preeclampsia and eclampsia compared to women who attended antenatal care. This finding was supported by another study [36]. Consistent with this finding, mothers who attended fewer than four ANC visits were at an increased risk of developing preeclampsia and/or eclampsia compared to mothers who attended four or more ANC visits [35]. Long distances to health facilities, limited knowledge about ANC services, and low socioeconomic status may be responsible for postnatal mothers failing to meet the WHO recommended four or more ANC visits; a situation that may lead to obstetric complications.
Women who did not attend school were at higher risk for preeclampsia and eclampsia compared to women who attended college or university. In line with this finding, another study conducted in Ethiopia reported that women with no formal education were 2.5 times more likely to develop preeclampsia and eclampsia compared to women who had attended secondary and above education [37]. Women with little formal education were less likely to seek medical attention and attend ANC until complications arose [34]. Educating women may help to expose women to more health education messages and campaigns, thereby enabling them to recognize danger signs and complications and take appropriate action during pregnancy [38]. The possible reasons could also be the difference in the literacy level of pregnant women and the difference in access to information, education, and communication. Women who had an early neonatal death had a higher risk of preeclampsia and eclampsia compared to women who did not have an early neonatal death. This finding was similar to another study that found that the risk of early neonatal death was four times higher for women with preeclampsia compared to non-pre-eclamptic women [39]. In line with this finding, another study in Ghana in 2017 found that the frequency of early neonatal deaths (3.8%) was generally high in women having HDPs, with the highest frequency occurring in the pre-eclamptic group [40]. The finding of significant adverse perinatal outcomes in women with HDPs indicates the importance of optimal antenatal, careful intrapartum, and adequate newborn care. This is important because adverse perinatal outcomes are a key indicator of maternal health and a reflection of the quality of obstetric and pediatric care.
Housewife women had a higher risk of preeclampsia and eclampsia compared to employed women. This result was consistent with another study that showed that women who were employed had 1.8 times higher odds of preeclampsia or eclampsia compared to those women who had other occupations, whereas those who had an occupation as housewives had two times higher odds of preeclampsia compared to those women who had other occupations [39]. Consistent with this, another study conducted in Cameroon found that the odds of HDP were three times higher among women who were housewives compared to women who were employed [41].
Policy and clinical practices: the information on the identified risk factors in this study should be communicated widely among maternity care providers in the region so that these risks are detected and controlled early in pregnancy. It is critical to stratify women for preeclampsia and eclampsia risk and to use biomarkers for early detection. In this study, preeclampsia and eclampsia were attributed to women who did not attend school. This could be reduced by improving educational intervention on pregnancy complications correlated with improved maternal health care utilization. Although, they evaluate the inequity of risky behavior between and within regions of Ethiopia in the use of maternal health services. In this study, preeclampsia and eclampsia were associated with women who had low wealth status. This could be reduced by improving women´s autonomy to decide on their healthcare services and supporting their willingness to take care of their health care needs. Moreover, interventions on household income and women´s education were key drivers of maternal mortality reduction and increased uptake of maternal health care services. In this study, preeclampsia and eclampsia were associated with women who resided in rural areas compared to urban residents. This could be prevented by focusing on geographical disparities in terms of urban/rural differences in the use of maternal health services. This should be addressed by actively mobilizing the communities to raise awareness and use of maternal health services. There is also a need to develop strategies to prevent, detect, and timely treat preeclampsia and eclampsia in rural populations. Women who did not attend antenatal care visits in the previous pregnancy were responsible for occurrence of preeclampsia and eclampsia. Educating women on the attendance of ANC visits and counseling on the signs and symptoms of pregnancy complications such as preeclampsia and eclampsia in the antenatal care clinic of the Sidama region may reduce this issue.
Strengths and limitations: one strength that could be linked to this study was selection bias was minimized by using the nested case-control study design compared to the traditional case-control study. One limitation could be recall bias linked to gestational age, which was calculated based on the women´s recall of their last menstrual period. However, women who could not remember the approximate gestational age were given an ultrasound scan. Social desirability could have been present because data were collected in face-to-face interviews, which could have led to socially acceptable answers. This study is not generalizable as it was limited to one region of the country, and it was limited to women who received hospital care.
In this study, a higher risk for preeclampsia and eclampsia was observed among women with low wealth status, those living in rural areas, younger women, women who had early neonatal death, women who did not attend antenatal care visits in the past pregnancy, women who did not attend formal education, and housewife women. Some of these factors could be positively influenced by educational interventions. Maternal and child health providers should screen pregnant women at risk for preeclampsia and eclampsia using these factors. Findings of this study will provide epidemiological evidence for policy makers and implementers to reduce the occurrence of preeclampsia and eclampsia using these factors.
What is known about this topic
- Previous studies in southern Ethiopia revealed inconsistencies in reporting factors associated with preeclampsia and eclampsia. Most studies did not include control groups or measure the risk of outcomes of interest and there were missing socio-demographic variables such as maternal education level.
What this study adds
- Women who did not attend school were three times more likely to develop preeclampsia and eclampsia [AOR: 3.00,95% CI: 1.10-8.19] compared to women who attended college/university.
- Women with a low wealth status were 98% [AOR: 1.98, 95% CI: 1.34-2.92] more likely to develop preeclampsia and eclampsia compared to women with a high wealth status.
- Women who resided in rural areas had a 75% [AOR: 1.75, 95% CI: 1.02-3.01] higher risk of preeclampsia and eclampsia compared to women who resided in urban areas.
The authors declare that they have no competing interests.
Author´s contributions: This study was carried outby all authors collaboratively. BJ, MA and KA contributed to conceptualizing and designing the study, curating and analyzing data, and writing the first draft. Also, BJ, MA, TA, and KA managed the investigation, literature searches, methodology review, writing, and contributed to data collection; BJ, MA, TA, and KA contributed to the manuscript review, resource, preparation, and editing. All the authors have read and agreed to the final manuscript.
We thank University of Gondar, Institute of Public Health, College of Medicine and Health Sciences for providing us the opportunity to conduct this study. We also thank supervisors for reviewing and editing the report of this PhD project. We thank Dorothy L. Southern for her critical review of this paper and for her support in editing and training in scientific writing. We also thank study participants who participated in this study.
Table 1: socio-demographic and obstetrics characteristics of women with preeclampsia/eclampsia and controls in Sidama region, southern Ethiopia, 2022
Table 2: maternal and fetal characteristics of women with preeclampsia/eclampsia and controls in Sidama region, southern Ethiopia, 2022
Table 3: bivariable and multivariable binary logistic regression analyses on risk factors for preeclampsia and eclampsia in Sidama region, southern Ethiopia, 2022
Figure 1: flow-diagram of the overall study process in Sidama region, southern Ethiopia, 2022
- Marciano Anselmini, Lucas Kreutz Rodrigues, Bruna Balestrin, Daniel de Paula Santana, Gisely Freitas, Leonardo Kreutz Rodrigues et al. Perinatal outcome of hypertensive pregnant women is related to the severity of preeclampsia. Clin Biomed Res. 2018;38(2):116-22. Google Scholar
- Townsend R, O´Brien P, Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integrated blood pressure control. 2016;9:79-94. PubMed | Google Scholar
- Amanual Getnet Mersha, Tadesse Melaku Abegaz, Mohammed Assen Seid. Maternal and perinatal outcomes of hypertensive disorders of pregnancy in Ethiopia: systematic review and metaanalysis. BMC Pregnancy and Childbirth. 2019 Dec 3;19(1):458. PubMed | Google Scholar
- Berhe AK, Kassa GM, Fekadu GA, Muche AA. Prevalence of hypertensive disorders of pregnancy in Ethiopia: a systemic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):34. PubMed | Google Scholar
- 3. Maternal Health Task Force. Nutrition and pre-eclampsia risk factors in Ethiopia. 2018. Accessed Mar 22, 2022.
- Firoz T, Sanghvi H, Merialdi M, von Dadelszen P. Pre-eclampsia in low- and middle-income countries. Best Practice Research Clinical Obstetrics Gynecology. 2011;25(4):537-48. PubMed | Google Scholar
- Schrauben SJ, Wiebe DJ. Health literacy assessment in developing countries: a case study in Zambia. Health Promot Int. 2017;32(3):475-81. PubMed | Google Scholar
- Musarandega R, Nyakura M, Machekano R, Pattison R, Munjanja SP. Causes of maternal mortality in sub-Saharan Africa: a systematic review of studies published from 2015 to 2020. J Glob Health. 2021;11:04048. PubMed | Google Scholar
- Negash Wakgar, Dubale Dulla, Deresse Daka. Maternal near misses and death in southern Ethiopia: a retrospective study. Ethiopian Journal of Reproductive Health. 2019;11(2). Google Scholar
- Mastewal Arefaynie Temesgen. Factors associated with hypertensive disorder of pregnancy in Kombolcha. Clinics Mother Child Health. 2017;14(4). Google Scholar
- Bryan Shaw, Agbessi Amouzou, Nathan P Miller, Jennifer Bryce, Pamela J Surkan, Nathan P Miller. A qualitative exploration of care-seeking pathways for sick children in the rural Oromia region of Ethiopia. BMC Health Services Research. 2017 Mar 9;17(1):184. PubMed | Google Scholar
- Ayele GLS, Agedew E. Factors associated with hypertension during pregnancy in Derashie Woreda South Ethiopia, case control. Quality in Primary Care. 2016;24(5):207-13. Google Scholar
- Hinkosa L, Tamene A, Gebeyehu N. Risk factors associated with hypertensive disorders in pregnancy in Nekemte referral hospital, from July 2015 to June 2017, Ethiopia: case-control study. BMC Pregnancy Childbirth. 2020 Jan 6;20(1):16. PubMed | Google Scholar
- Larry Jones WT, Wisdom Kudzo Axame, Richard Owusu, Phyllis Atta Parbey, Elvis Tarkang Mohammed Takase et al. Risk factors associated with pregnancy induced hypertension in the Hohoe Municipality of Ghana. J PrevMed Healthc. 2017;1(3):1011.
- Gilles Guerrier, Bukola Oluyide, Maria Keramarou, Rebecca F Grais. Factors associated with severe preeclampsia and eclampsia in Jahun, Nigeria. Int J Womens Health. 2013 Aug 19;5:509-13. PubMed | Google Scholar
- Maru Mekie, Dagne Addisu, Minale Bezie, Abenezer Melkie, Dejen Getaneh, Wubet Alebachew Bayih et al. Knowledge and attitude of pregnant women towards preeclampsia and its associated factors in South Gondar Zone, Northwest Ethiopia: a multi-center facility based cross-sectional study. BMC Pregnancy Childbirth. 2021 Feb 23;21(1):160. PubMed | Google Scholar
- Badego B, Yoseph A, Astatkie A. Prevalence and risk factors of hypertension among civil servants in Sidama Zone, south Ethiopia. PLoS ONE. 2020;15(6):e0234485. PubMed | Google Scholar
- Hiwot Abera Areru, Mesay Hailu Dangisso, Bernt Lindtjørn. Births and deaths in Sidama in southern Ethiopia: findings from the 2018 Dale-Wonsho health and demographic surveillance system (HDSS). Glob Health Action. 2020 Dec 31;13(1):1833511. PubMed | Google Scholar
- Goals and targets. Final list of proposed sustainable development goal indicators. 2016.
- IPHCE. Health Sector Transformation Plan II HSTP II 2020/21-2024/25 Ministry of Health Ethiopia. 2021.
- Eshetu Seyom, Mubarek Abera, Million Tesfaye, Netsanet Fentahun. Maternal and fetal outcome of pregnancy related hypertension in Mettu Karl Referral Hospital, Ethiopia. Journal of Ovarian Research. 2015;8(10). PubMed | Google Scholar
- Mulugeta Shegaze, Yohannes Markos, Wubeshet Estifaons, Iyasu Taye, Erkihun Gemeda, Tigist Gezahegn et al. Magnitude and associated factors of preeclampsia among pregnant women who attend antenatal care service in public health institutions in Arba Minch Town, Southern Ethiopia, 2016. Gynecol Obstet (Sunnyvale). 2016;6(12):1000419. Google Scholar
- Tesfaye Abera Gudeta, Tilahun Mekonnen Regassa. Prevalence of pregnancy induced hypertension and its bad birth outcome among women attending delivery Service. J Preg Child Health. 2017;4(5).
- Lemi Belay Tolu, Endale Yigezu, Tadesse Urgie, Garumma Tolu Feyissa. Maternal and perinatal outcome of preeclampsia without severe feature among pregnant women managed at a tertiary referral hospital in urban Ethiopia. PLoS ONE. 2020;15(4):e0230638. PubMed | Google Scholar
- Obstetrics management protocol for hospitals. MOH, Ethiopia. 2021.
- Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. 2018 Jul;13:291-310. PubMed | Google Scholar
- Frank I Olotu, Michael J Mahande, Jenny Renju, Joseph Obure. Prevalence and risk factors for preeclampsia/eclampsia in Northern Tanzania. Journal of Public Health and Epidemiology. 2020;12(2):78-85. Google Scholar
- Birhanu Jikamo, Mulat Adefris, Telake Azale, Kassahun Alemu. Cultural adaptation and validation of the Sidamic version of the World Health Organization Quality-of-Life-Bref Scale measuring the quality of life of women with severe preeclampsia in southern Ethiopia, 2020. 2021 Oct 12;19(1):239. PubMed | Google Scholar
- Kelemu Tilahun Kibret, Catherine Chojenta, Ellie D'Arcy, Deborah Loxton. The effect of dietary patterns on hypertensive disorders of pregnancy in North Shewa, Ethiopia: a propensity score matched case-control study. Pregnancy Hypertension. 2020 Oct;22:24-29. PubMed | Google Scholar
- Liew Lee Chan, Noraini Idris. Validity and reliability of the instrument using exploratory factor analysis and Cronbach´s alpha. International Journal of Academic Research in Business and Social Sciences. 2017;7(10). Google Scholar
- David W Hosmer, Stanley Lemeshow. Applied logistic regression second edition. 2013. Google Scholar
- Delelegn Emwodew Yehualashet, Binyam Tariku Seboka, Getanew Aschalew Tesfa, Tizalegn Tesfaye Mamo, Elias Seid. Determinants of optimal antenatal care visit among pregnant women in Ethiopia: a multilevel analysis of Ethiopian mini demographic health survey 2019 data. Reproductive Health. 2022 Mar 5;19(1):61. PubMed | Google Scholar
- Yitagesu Belayhun, Yibeltal Kassa, Niguse Mekonnen, Wakgari Binu, Mahilet Tenga, Bereket Duko. Determinants of pregnancy-induced hypertension among mothers attending public hospitals in Wolaita Zone, South Ethiopia: findings from unmatched case-control study. Hindawi International Journal of Hypertension. 2021 (Article ID 6947499):9. Google Scholar
- Mwanri AW, Kinabo JL, Ramaiya K, Feskens EJ. High blood pressure and associated risk factors among women attending antenatal clinics in Tanzania. J Hypertens. 2015;33(5):940-7. PubMed | Google Scholar
- Gorbee G Logan, Peter K Njoroge, Lambert O Nyabola, Marshal M Mweu. Determinants of preeclampsia and eclampsia among women delivering in county hospitals in Nairobi, Kenya. F1000 Research. 2022;9(192). Google Scholar
- Mengesha H, Lerebo W, Kidanemariam A, Gebrezgiabher G, Berhane Y. Pre-term and post-term births: predictors and implications on neonatal mortality in Northern Ethiopia. BMC Nurs. 2016 Aug 5;15:48. PubMed | Google Scholar
- Mekonen L SZ, Wubshet E, Haile S. Pregnancy induced hypertension and associated factors among pregnant women in Karamara Hospital, Jijiga, Eastern Ethiopia. J Preg Child Health. 2015;5(379).
- Sripad P, Ismail H, Dempsey A, Kirk K, Warren CE. Exploring barriers and opportunities for pre-eclampsia and eclampsia prevention and management in Ethiopia. 2018. Google Scholar
- Abalos E, Cuesta C, Carroli G, Qureshi Z, Widmer M, Vogel JP et al. Pre-eclampsia, eclampsia and adverse maternal and perinatal outcomes: a secondary analysis of the World Health Organization Multicounty Survey on Maternal and Newborn Health. BJOG. 2014;121(Suppl. 1):14-24. PubMed | Google Scholar
- Kwame Adu-Bonsaffoh, Michael Y Ntumy, Samuel A Obed, Joseph D Seffah. Perinatal outcomes of hypertensive disorders in pregnancy at a tertiary hospital in Ghana. BMC Pregnancy Childbirth. 2017 Nov 21;17(1):388. PubMed | Google Scholar
- Tebeu PM, Foumane P, Mbu R, Fosso G, Biyaga PT, Fomulu JN. Risk factors for hypertensive disorders in pregnancy: a report from the Maroua regional hospital, cameroon. J Reprod Infertil. 2011 Jul;12(3):227-34. PubMed | Google Scholar