Prevalence and associated factors of anaemia among antenatal care attendants in the Kintampo municipality
Issah Sumaila, Alexander Manu, Mustapha Hallidu
Corresponding author: Issah Sumaila, Department of Public Health, Kintampo Municipal Hospital, Ghana Health Service, Bono East, Ghana 
Received: 01 Sep 2022 - Accepted: 25 Oct 2022 - Published: 03 Nov 2022
Domain: Infectious diseases epidemiology, Obstetrics and gynecology, Public health
Keywords: Anemia, pregnant women, prevalence
©Issah Sumaila et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Issah Sumaila et al. Prevalence and associated factors of anaemia among antenatal care attendants in the Kintampo municipality. PAMJ-One Health. 2022;9:16. [doi: 10.11604/pamj-oh.2022.9.16.37122]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/9/16/full
Research 
Prevalence and associated factors of anaemia among antenatal care attendants in the Kintampo municipality
Prevalence and associated factors of anaemia among antenatal care attendants in the Kintampo municipality
&Corresponding author
Introduction: anemia among pregnant women is endemic in developing countries and often results in complications to mother and fetus (born or unborn). WHO estimates showed that Sub-Saharan Africa has the highest prevalence of 57.1%. Objectives: to determine the prevalence of anemia and its associated factors among pregnant women attending Kintampo North Municipal Hospital (KNMH).
Methods: a cross-sectional study in which data were collected among 496 pregnant women who attended KMH between May 2019 and June 2019. Structured questionnaires were used to obtain information on sociodemographic factors, medical history, and key dietary consumption patterns of participants. The main outcome of interest is anemia in pregnancy, defined as a hemoglobin concentration of less than 11.0g/dl. Test of associations between independent variables and the dependent variable was examined using Chi-square. Binary logistic regression models were also fitted after dichotomizing the outcome by whether the women had anemia or not. Statistical significance level was assessed at 5% and 95% confidence interval.
Results: the prevalence of anemia was moderate. It is associated with these factors: basic education (tripled the risk), farming/trading reduced the risk by 94%, birth interval of less than 2 years doubled the risk, parity (primiparous and multiparous) doubled the risk, fruits and vegetables consumption (once and twice a week) tripled the risk and pica consumption among the pregnant women doubled its risk.
Conclusion: prevalence of anemia was high. Pica, educational level, occupation, and frequency of intake of fruits and dark green leafy vegetables were associated with anemia among pregnant women.
Pregnancy is a joyous and rewarding period in a woman's life. It is not just a matter of waiting to give birth. It can also be a source of pain and sorrow when complications and unforeseen situations jeopardize the pregnancy, resulting in illness or even death [1]. Anemia is among the conditions that could take away the joy of a couple. During normal pregnancy, blood increases in volume, leading to concomitant hemodilution [2]. Though red blood cell (RBC) increases in mass during pregnancy, plasma volume increases more, resulting in a relative anemia [3]. This increase in plasma volume compared to the increase in RBC results in a physiologically lowered hemoglobin (Hb) level [2]. The demand for iron in the blood increases with pregnancy. This leads to an increased risk of iron deficiency in pregnant women, with 57.1% of them in developing countries suffering from anemia [4]. This increased iron demand leads to an increased risk of iron deficiency in pregnant women, with 57.1% of them living in developing countries [5]. Anemia is directly responsible for more than 3% of maternal mortality in Africa [6]. Pregnancy anemia lowers blood loss tolerance, resulting in decreased function and heart failure [7]. An estimated 56 million pregnant women are anemic across the globe [8]. Anemia in pregnancy is estimated to affect 41.8 percent of pregnant women worldwide, with rates ranging from 5.7 percent in the United States to 75 percent in the Gambia [9]. Anemia affects half a billion women of reproductive age worldwide [8]. In 2011, anemia affected 29 percent of non-pregnant women and 38 percent of pregnant women aged 15-49 years globally, with the highest frequency in South Asia and Central and West Africa [10].
About 50% of reported cases of anemia in developing countries are related to nutritional anemia, and they occur because of insufficient iron intake [11]. Diet restriction, growth spurt, and heavy blood loss in any form are risk factors for developing anemia [12]. Age, ethnicity, parity, and education are but a few of the many determinants of anemia in pregnant women [13]. Different parts of the world have varying estimates for the prevalence of maternal anemia. Reports from developed countries indicate 2.0% - 45.0% of pregnant women are diagnosed with anemia, which is generally higher in developing countries (5.0% - 90.0%) [9]. The worldwide prevalence of anemic pregnant women according to the World Health Organization (WHO) report was 55.9% [14]. India was reported to have a prevalence range of 33.0% - 8.0% [9]. The highest prevalence of maternal anemia was recorded in sub-Saharan Africa (SSA). However, it is declining slowly over the years compared to other regions [15]. WHO classifies maternal anemia to be mild if the prevalence is 5-19%, moderate if the prevalence is 20-39%, and severe if it is at least 40% [16]. According to a WHO report, 62% of pregnant women in Ghana have a blood Hb concentration of less than 11.0 g/L, and this according to the same report is an issue of severe public health importance [17]. A multinational study that compared anemia prevalence, access, and usage of antenatal care and anemia reduction interventions, estimated the maternal prevalence of anemia in Ghana to be 70%, this figure exceeds the WHO “severe public health problem” threshold [16].
Studies across the globe demonstrate a high prevalence of anemia in pregnancy [18]. In Ghana, the most common condition pregnant women present with at the health facilities is anemia. This may increase morbidity and mortality in both pregnant women and their unborn or newly born children. In the year 2003, the Ghana Health Service (GHS) estimated that 200 women died during pregnancy and childbirth due to anemia-related complications [19]. In 2014, 45 percent of pregnant women in Ghana were anemic [20] and by 2017, the prevalence of anemia reduced to 29 percent [21]. None of such studies have been conducted in the Kintampo North Municipality to estimate the prevalence of maternal anemia and its associated factors. Therefore, to bridge this gap, the present study was designed to estimate the prevalence of anemia at registration and current ANC visit, demographic characteristics of those at risk, and the major causes in support of interventions to decrease the incidence of anemia in pregnant women within the Kintampo North Municipality in the Bono East of Ghana.
Study area: the study was conducted in the Kintampo North Municipality (KNMH) in the Bono East Region of Ghana. The Municipality is one of the 11 municipals/districts in the Bono East Region with a total population of 111,448 [22] and an estimated pregnancy of 4,534 for the year 2017. It has an average annual ANC registrant of 4,968, a monthly gestation of 36 weeks, and an ANC attendant of more than 278. The Kintampo North Municipality has 17 facilities offering antenatal care, 3 of which are in urban settings and the rest are in a rural setting. Facilities in urban settings comprise a government hospital (KNMH), 2 private hospitals, and 1 private maternity home. Facilities in rural settings included 4 health centers (H/C) and 10 community-based health planning and services (CHPS) compounds (KMHD, 2017). The facility provides these services: ANC, inpatient, OPD, pharmacy, X-ray, laboratory, mortuary, maternity, and theatre. The hospital has 87 beds, with an average outpatient case of 400 per day. The number of nurses is 134 and 3 doctors with 8 physician assistants. There are 12 midwives, 4 community health nurses, a dietician, and 2 nutrition officers.
Study design: this was a hospital-based cross-sectional study that was carried out at the KNMH from August 2018 to June 2019.
Sample population: all pregnant women aged 15 to 49 years who visited the antenatal clinic at the Kintampo Municipal Hospital during the study period.
Inclusion and exclusion criteria: the inclusion criteria include all pregnant women of all trimesters, with at least two records of Hb, women who are of a sound mind or without any major illness, and who consent to voluntarily participate in this study. On the other hand, the exclusion criteria include; pregnant women who attended ANC for the first time, women who were transfused in less than two weeks because the result will not be a true reflection of their normal Hb, pregnant women that were sick and unable to respond to the questionnaire, pregnant women without a checked Hb, women who are mentally sound and women who did not consent to participate in the study.
Sample size calculation, sampling techniques and procedures: the sample size for the study was calculated using the Snedecor & Cochran, (1989) formula [23]:
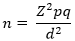
where n = sample size; z = z-score that corresponds with 95% confidence interval = 1.96; p = estimated proportion of anemia in pregnancy = 29% = 0.29, [21], q = 1-p = 1 - 0.29 = 0.71, d = Margin of error = 5% = 0.05. From above, the minimum sample size for this study was estimated as 349. Data were collected from 496 participants from May 2019 to June 2019 using simple random sampling. Twenty-five participants were selected daily from an average of 90 antenatal attendants. The total number of women was counted and pieces of paper were cut and numbered to match the exact number of women present at the commencement of ANC services. Twenty-five of these papers were labeled "yes" while the rest were labeled "no". Each participant was asked to pick a piece of paper. Those who picked the pieces of paper with "yes" as the label were enrolled in the study after they had duly consented and signed to partake in the study. Pregnant women that reported to the ANC as first-time registrants were not enrolled in the study and those that did not consent to be part of the study after they picked "yes" were excused. The process was repeated until the maximum number of twenty-five participants was reached for each day. Nurses were then requested to direct every eligible participant that came from the laboratory with hemoglobin (Hb) test results to a secluded place that ensured privacy for the respondent. This secluded place was not too far from the point of care. This procedure was repeated for six weeks until a sample of four hundred and ninety-six (496) for the study was obtained.
Data collection tools and procedures: the questionnaire was pretested at Yizura hospital in the Kintampo North Municipality. Data were extracted from the ANC booklets on basic socio-demographic characteristics and two hemoglobin levels of pregnant women in any available trimester. The questionnaire was adapted from a study and modified to suit the study participants and the study site [24]. A suitably structured questionnaire for the quantitative cross-sectional study was used to obtain information on demographic characteristics, socioeconomic background, medical, and dietary practices of the study participants. Pretesting of the questionnaire was carried out to check for its validity in the local context before the actual data collection commenced.
Data processing, analysis, and presentation: data were entered and cleaned in Microsoft Excel version 2016. It was then exported to Stata version 15 for analysis. Descriptive statistics (means and standard deviations) were calculated for continuous variables, while proportions were used to calculate categorical variables. Numerical variables like age of participants, gravidity, parity, number of ANC visits, Hb levels at both first and current visits, gestational age at first, and current ANC visits were all recorded and categorized. The age of participants was categorized (15-19, 20-24, 25-29, 30-34, 35-39, and ? 40). Gestational ages at first and current ANC visits were both categorized into first (1 to 12 weeks), second (13 to 27 weeks), and third trimesters (28 weeks to birth). Moreover, Hb at first and current ANC visits were categorized into no anemia (Hb ? 11g/dl), mild anemia (Hb 10 - 10.9g/dl), moderate anemia (7 - 9.9g/dl), and severe anemia (Hb < 7g/dl). These were further re-categorized into anemia and no anemia (WHO, 2001). Chi-square analysis was then used to test for the association between categorical variables, with the re-categorized Hb at the current ANC visit as the main dependent variable. Binary logistic regression analysis was used to assess for socio-demographic, socioeconomic, clinical, and dietary factors that were associated with anemia. Statistically significant factors (P-value <0.05) were included in the multivariable logistic regression models.
Ethical consideration: Approval for the study was granted by Ghana Health Service Ethics Review Committee. The reference number for the study is GHS-ERC 042/04/19. Management of hospital gave permission before the study commenced. The study procedures and confidentiality were all explained to respondents in a language they understood. Only respondents who signed the inform consent forms were allowed to participate in the study.
Sociodemographic characteristics of the women: Table 1 shows the sociodemographic characteristics of respondents. The age of the pregnant women ranged from 15 to 43 years, with a mean (SD) of 28.3 ± 5.6 years. Most women (83.7%) were within the ages of 20 - 35 years, 402(81.1%) were married, and 295(59.5%) were Christians. The majority (60.5%) of the respondents were self-employed and about 91.3% had some level of education. A total of 141 (28.4%) of the pregnant women were primiparous and, 351(70.8%), had at most 5 family members in their household.
Clinical characteristics of women: the majority (70%) of the respondents had a birth spacing interval of two or more years, while 20(4.0%), reported less than two years of birth interval. During their first ANC visit, 277(55.9%) of the women were found to be in their first trimester. The current ANC visit, however, had the majority, 438(88.3%), of the women in their third trimester. At the time of the interview, 442(89.1%) of them made at least four different ANC visits. Of the majority, 155(31.3%) were pregnant for the second time, while 206(41.5%) had given birth to two or more children (Table 2).
Consumption of iron supplements and iron-rich foods: Table 3 displays iron supplementation and consumption of iron-rich foods. Almost all, 491(99.0%) pregnant women received iron supplements, 370(75.1%) of them received it on more than two occasions, and 489(98.6%) took it always. The number of participants who consumed eggs/meat more than two times per week was 364(73.4%), while 326(65.7%) of them consumed fruits and or vegetables more than two times in a week. Most, 413(83.3%), of the pregnant women did not consume non-food items (pica).
Medical interventions and malaria infection in pregnant women: out of the 496 women that were recruited into the study, the majority (63.3%) of them received Insecticide-Treated Nets (ITN), 302(60.9%) slept under the ITN the previous night and 140(28.2%) of them were infected with malaria parasites. Most, 438(88.3%) of the participants received malaria prophylaxis (SP) and 274(55.2%) were given anti-helminths during pregnancy (Table 4).
Prevalence of anemia among antenatal attendants
Severity of anemia among antenatal attendants: of the 496 pregnant women, 1(0.2%) of them presented with severe anemia (Hb<7g/dl), 62(12.5%) had moderate anemia (Hb 7 - 9.9 g/dl) and 108(21.8%) had mild anemia (Hb 10 - 10.9 g/dl). At the current ANC visit, the differential prevalence of anemia among the 496 pregnant women were as follows: 36(7.3%) had moderate anemia and 105(21.2%) had mild anemia.
Anemia status among antenatal attendants: among the 496 pregnant women that were recruited into the study from the ANC, the prevalence of anemia (Hb <11g/dl) in the Kintampo North Municipal Hospital at registration and at current gestation were found to be 34.5% (95% CI = 30.4 - 38.8) and 28.4% (95% CI = 24.6 - 32.6) respectively.
Changes in hemoglobin levels of pregnant women atr and current gestation: the mean Hb of the pregnant women at registration and current ANC visits were respectively 11.36g±1.29g/dl and 11.44±1.05g/dl. The Hb increased by 0.08g/dl from registration to current gestation while pregnant women were given interventions like SP, IFA, ITNs and anti-helminths. The change was not statistically significant (p=0.169). Detail is in Table 5.
Bivariate association between sociodemographic characteristics of women and current anemia Status: there was a significant association between anemia status at the current visit and level of education (χ2=22.42, P=0.001). The study further established that the association between the occupation of pregnant women and anemia was significant (χ2=27.37, p=0.001). However, there was no association between current anemia status and marital status (χ2 =2.91, p=0.23) and age (χ2 =6.25, p=0.28). (Table 1).
Bivariate association between clinical characteristics of women and current anemia status: there was a significant association between the number of ANC visits and anemia among pregnant women (Chi = 6.32, p=0.04). The majority, 442(89.1%), of the women made four or more ANC visits and 16(47.1%) of them were anemic. No association was observed between current anemia status and other clinical characteristics measured in this study (Table 2).
Bivariate association between consumption of iron supplements and iron-rich foods with current anemia status: a significant association was established between the frequency of vegetables/fruits consumption and anemia status of the pregnant women (χ2=23.68 p=0.001). About three-fourths, 256(72.1%), of those who consumed vegetables/fruits two times or more in a week were normal. Almost half, 96(49.7%), of those who presented with anemia, also consumed it more than two times a week. Pica consumption was significantly associated with anemia in pregnant women (χ2 =14.76, p=0001). A little over one-third, 38(27.0%), of those who had anemia craved for pica, while the majority of those with normal Hb, 310(87.2), did not. Details are in Table 3.
Bivariate association between medical intervention and anemia in pregnant women: none of the medical interventions were significantly associated with anemia among pregnant women, although it is expected to reduce the burden of maternal anemia (Table 4)
Logistic analysis of all the factors that associated with anemia in pregnant women: level of education was significantly associated with anemia. The odds of pregnant women with basic education being anemic were 2.96 times compared to pregnant women with no formal education (cOR: 2.96, [95%CI: 1.23 - 7.0]; p=0.015). There was a significant association between occupations with anemia. The odds of a self-employed pregnant woman becoming anemic were 85% less compared to a pregnant government employee (cOR: 0.15,95% CI: 0.04 - 0.48]; p=0.002). Again, the odds of an unemployed pregnant woman becoming anemic were 79% less compared to a pregnant government employee (cOR: 0.21, [95%CI: 0.06 - 0.73]; p=0.014). Finally, the odds of pregnant women who were neither self-employed nor unemployed (predominantly students) becoming anemic were 96% less compared to a pregnant government employee (cOR: 0.04, [95%CI: 0.10 - 0.16]; p=0.001). Birth spacing, gestational age at first and second ANC visit, gravidity, parity, and several ANC visits were all not associated with anemia during univariate logistic analysis. However, after adjusting for the age of pregnant women, birth spacing and parity become significantly associated with anemia. The odds of pregnant women with a birth spacing of less than 2 years being anemic were 1.89 compared to women with a birth spacing of 2 years and above (aOR: 1.89, [95% CI: 0.76 - 1.89]; p=0.018). Similarly, the odds of primiparous women having anemia were 1.32 times compared to nulliparous women (cOR:1.32, [95% CI: 0.79 - 2.20], p=0.28) and (aOR: 1.78, [95% CI: 1.03 - 3.06]; p=0.04). The odds of multiparous women becoming anemic were 1.08 times compared to nulliparous women (cOR: 1.08, [95%CI: 0.67 - 1.74]; p=0.75) and (aOR: 2.16, [95%CI: 1.16 - 4.01]; p=0.02). There was a significant association between the consumption of vegetables/fruits with pregnant women being anemic or not. The odds of pregnant women who consumed vegetables/fruits twice a week becoming anemic was 2.58 times compared to those who did not consume it (aOR: 2.58, [95%CI: 1.3 - 8.7]; p=0.016). Pregnant women who consumed pica were 2.54 times more likely to become anemic, compared to those that did not consume it (cOR: 2.54, [95CI: 1.56 - 4.13]; p=0.001) and (aOR: 2.37, [95CI: 1.45 - 3.87]; p=0.001) (Table 6).
This study was a hospital-based cross-sectional study that was conducted to estimate the prevalence of anemia and its associated factors among pregnant women who visited the KNMH. There was a drop in the prevalence of anemia from the first ANC visit to the current visit, 34.5% to 28.4%, respectively. The 6.1% drop in the level of anemia may be attributed to ANC services like the provision of insecticide-treated nets (ITN), iron supplements, malaria prophylaxis, and counselling among others that were rendered to pregnant women. Malaria in pregnancy is a common cause of poor maternal and child development outcomes. It can trigger various health complications such as birth defects, miscarriages, low birth weight, and maternal anemia [25]. To decrease the prevalence of malaria in Ghana, intermittent preventive treatment with sulfadoxine pyrimethamine (SP) is being implemented in all hospitals and clinics. The majority of the pregnant women in this study have received SP and have not been affected by malaria. Although malaria prevalence was not assessed in the current study, it can be said that the use of SP could reduce maternal anemia and adverse birth outcome [26]. Moreover, 16.7% of women practice pica. Pica is often referred to as craving for or ingesting non-food things such as a chewing stick, clay, chewing gum, cola nuts, etc to prevent nausea and vomiting. Various studies have associated this phenomenon with iron deficiency anemia [27,28]. To avoid unintended pregnancy outcomes, it is critical that counselling on this topic be increased in ANCs.
The current prevalence of anemia among ANC attendants was estimated at 28.4%. Moreover, the severity (severe, moderate, and mild) of anemia reduced at the current visit compared to the first and this can be attributed to the ANC services that were offered to the pregnant women. In comparing the findings of the current study with other studies in Ghana, it was observed that the estimated prevalence of anemia is lower than 34.4% anemia prevalence reported in Sekondi-Takoradi [29], 50.8% in Tamale Teaching Hospital in Northern Ghana [30], 56.5% in Ashanti Region [31], 57.1% in Sekyere West in southern Ghana [32] and 70.0% in twenty-five rural communities in Northern Ghana [33]. In comparison with similar studies in Africa and elsewhere, the prevalence of anemia in the current study is lower than 54.5% in Nigeria [34], 42.7% in South Africa [35], 51.3% in rural Egypt [36], 57.0% in Kenya [37], 57.7% in Lahore, Pakistan [38] and 59.0% in India [39]. This low prevalence of anemia in this study could be attributed to the specific interventions put in place at the Kintampo Municipal Hospital. These included daily health education of pregnant women at the ANC on the causes of anemia in pregnancy and how to prevent it or mitigate its effect on those who were already affected. Again, those with anemia were linked to the public health unit to be given nutritional counselling by the registered dietician or nutrition officers. This proper coordination of the antenatal care staff and public health unit together with socio-economic status could be attributed to the differences. Moreover, the Kintampo Health Research Center (KHRC) has taken a lot of interventions on anemia in the municipality and has organized well-coordinated and timely education and sensitization on anemia. All these could be missing in the other areas and hence, the large differences. However, the prevalence of anemia in this study is higher than 21.3% in Addis Ababa, Ethiopia [40], 23.2% in Southern Ethiopia [41], and 22.1% in Gulu and Hoima Regional Hospital, Uganda [42]. These low prevalences may be associated with the study sites, socio-demographic characteristics, and socio-economic status of the study participants. Again, the above-listed countries are located in the Eastern part of Africa with better Gross Domestic Product (GDP) compared to Ghana except for Uganda.
The frequency of vegetables/fruits consumption was strongly associated with anemia in pregnant women. Those who consumed vegetables/fruits at least twice every week were less likely to have anemia compared to those who consumed only ones. This could be explained by the fact that dark green leafy vegetables contain some amount of non-haem iron that help to reduce the severity of anemia. Fruits such as oranges, limes, lemons, and tomatoes contain high amounts of vitamin C that increase the bioavailability of non-haem iron from vegetables and make their absorption much easier. The bioavailability of this non-haem is less compared to haem iron (iron from animal sources). Moreover, fruits contain vitamins like folate and cobalamin that help to prevent other forms of anemia. Other studies that were conducted in different countries showed similar trends [41,43,44]. A study conducted by [45], however, gave a contrasting result. These differences can be attributed to variation in the study sites, individual differences, and sampling techniques. The number of ANC visits by pregnant women was also significantly associated with anemia. However, it lost its significance after it was modelled in logistic regression. Various studies across the globe have also established a significant relationship between the number of ANC visits and becoming anemic [46-48]. Those with 4 or more ANC visits were less likely to be anemic because of the numerous interventions that were put in place to reduce their severity of anemia. Malaria prophylaxis, iron and folate supplements, ITNs, and nutritional counselling are some of the preventive measures that were put in place to reduce the prevalence of anemia among them. Women with the recommended number of 4 or more ANC visits were more likely to receive all above services compared to those with fewer visits.
Occupation of study participants was among the factors that were associated with anemia. Those who were self-employed and those who were unemployed (including housewives and concubines) were much less likely to be anemic compared to those working in government establishments. This can be attributed to the fact that those with formal employment may not have the time to eat regularly compared to those who are unemployed. Moreover, the housewives are those who prepare the meals and as such, they are better positioned to consume the juiciest parts. Again, those who were not working and not married may be concubines to other men. Studies have shown that men take care of their concubines better than their wives. Some studies that were conducted in Ghana and other countries like Ethiopia agree with this finding [49,50]. Other studies, however, did not find a significant association between occupation and becoming anemic [32,46]. Pica was significantly associated with anemia among pregnant women. Those who craved for pica were 2.54 times more likely to be anemic compared to those who did not. This was significant (p=0.001) in the linear logistic model and after adjusting. aOR 2.37(1.45 - 3.87), p=0.001. A study by [45], established an association between pica and anemia. Stephen et al., however, did not find a significant association between pica and anemia in their study [46]. These differences could be explained by the type of clay or soil the pregnant women crave for. This is because different soils/clays have different species of blood-sucking worms and their eggs. The prenatal Hb concentration of women may also play a key role in this regard.
Limitations of the study: the study was a cross-sectional study as such no casual-effect relationship could be established. The study participants included only ANC attendants, thus generalisation of these findings ought to be done with extreme caution. Also, women who might be attending ANC for the first time who might have substantially higher anemia may be excluded due to the design of the study.
Prevalence of anemia in pregnant women who participated in the study was high at registration and current ANC visits respectively. Pica, educational level, occupation, and frequency of intake of dark green leafy vegetables and fruits were associated with anemia among pregnant women. It is therefore important to continue health education on adequate intake of fruits and vegetables while encouraging the avoidance of pica, especially during pregnancy.
Funding: the research forms part of the requirement for the award of Master of Public Health degree of the first author at the School of Public Health, University of Ghana, and was fully funded by the first author.
What is known about this topic
- Neonates born to iron-deficient mothers: are more susceptible to anemia, are low birth weight or preterm and have higher mortality;
- The demand for iron in the blood increases with pregnancy;
- WHO classifies maternal anemia to be mild if the prevalence is 5-19%, moderate if the prevalence is 20-39%, and severe if it is at least 40%.
What this study adds
- Majority of the respondents had a birth spacing interval of two or more years;
- Pica, educational level, occupation, and frequency of intake of dark green leafy vegetables and fruits were associated with anemia among pregnant women;
- It is important to continue health education on adequate intake of fruits and vegetables while encouraging the avoidance of pica, especially during pregnancy.
The authors declare no competing interests.
Issah Sumaila conceptualized the study, designed the study protocols, performed the data analysis and the first draft of the manuscript´s introduction and methods. Alexander Manu and Mustapha Hallidu made inputs in the data analysis, discussed the results, conclusion and recommendations. All authors performed critical review of the manuscript and subsequently approved it. All the authors have read and agreed to the final manuscript.
We wish to extend our heartfelt gratitude to the management of Kintampo Municipal Hospital and all the participants that willingly consented to be part of this study.
Table 1: bivariate association between sociodemographic characteristics of women and current anaemia status
Table 2: bivariate association between clinical characteristics of women and current anaemia status
Table 3: bivariate association between consumption of iron supplements and iron-rich foods with current anaemia status (N=496)
Table 4: bivariate association between a medical intervention and current anaemia status
Table 5: hemoglobin levels of pregnant women at registration and at 36 weeks gestation
Table 6: logistic analysis of sociodemographic factors and its association with current anaemia status among pregnant women
- Nguyen TV, King J, Edwards N, Dunne MP. Under great anxiety: pregnancy experiences of Vietnamese women with physical disabilities seen through an intersectional lens. Soc Sci Med. 2021;114231. PubMed | Google Scholar
- Peace JM, Banayan JM. Anemia in pregnancy: pathophysiology, diagnosis, and treatment. Int Anesthesiol Clin. 2021 Jul 1;59(3):15-21. PubMed | Google Scholar
- Akinlaja O. Hematological changes in pregnancy - the preparation for intrapartum blood loss. Obs Gynecol Int J. 2016;4(3):00109. Google Scholar
- Abu Hashim H, Foda O, Ghayaty E. Lactoferrin or ferrous salts for iron deficiency anemia in pregnancy: a meta-analysis of randomized trials. Eur J Obstet Gynecol Reprod Biol. 2017 Dec;219:45-52. PubMed | Google Scholar
- Gebreweld A, Bekele D, Tsegaye A. Hematological profile of pregnant women at St. Paul´s Hospital Millennium Medical. BMC Hematol. 2018 Jul 9;18:15. PubMed | Google Scholar
- Yaya S, Anjorin SS, Adedini SA. Disparities in pregnancy-related deaths: spatial and Bayesian network analyses of maternal mortality ratio in 54 African countries. BMJ Glob Heal. 2021;6(2):e004233. PubMed | Google Scholar
- Anand IS, Gupta P. Anemia and iron deficiency in heart failure: current concepts and emerging therapies. Circulation. 2018;138(1):80-98. PubMed | Google Scholar
- WHO/CDC. Worldwide prevalence of anemia 1993-2005: WHO Global Database on anemia. Geneva, Switzerland: WHO Press; 2008.
- Tomar SG, Singhal S, Shukla A. Anemia in pregnancy: epidemiology and it´s determinants. Int J Med Heal Res. 2017;3(1):5-9.
- Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Heal. 2013;1(1):e16-25. PubMed | Google Scholar
- Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615-24. PubMed | Google Scholar
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014 Feb;19(2):164-74. PubMed | Google Scholar
- Gaillard R, Eilers PHC, Yassine S, Hofman A, Steegers EAP, Jaddoe WV. Risk factors and consequences of maternal anaemia and elevated haemoglobin levels during pregnancy: a population-based prospective cohort study. Paediatr Perinat Epidemiol. 2014 May;28(3):213-26. PubMed | Google Scholar
- WHO. Global nutrition targets 2025: anaemia policy brief (WHO/NMH/NHD/4). Glob Nutr Targets 2025. 2014;2(WHO/NMH/NHD/4):8. Accessed Sep 1, 2022.
- Adamu AL, Crampin A, Kayuni N, Amberbir A, Koole O, Phiri A et al. Prevalence and risk factors for anemia severity and type in Malawian men and women: Urban and rural differences. Popul Health Metr. 2017;15(1):1-15. PubMed | Google Scholar
- Klemm RD, Sommerfelt AE, Boyo A, Barba C, Kotecha P, Mona S et al. Cross-country comparison of anemia prevalence, reach, and use of antenatal care and anemia reduction interventions. Usaid. 2011;(July):1-88.
- WHO. The global prevalence of anaemia in 2011. Geneva World Heal Organ. 2015;48. Google Scholar
- Qureshi IA, Arlappa N, Qureshi MA. Prevalence of malaria and anemia among pregnant women residing in malaria-endemic forest villages in India. Int J Gynecol Obstet. 2014;127(1):93. PubMed | Google Scholar
- Ghana Statistical Service (GSS), Ghana Health Service (GHS), and ICF International. Ghana Demographic and Health Survey 2014. Rockville, Maryland, USA: GSS, GHS, and ICF International; 2015. Accessed Sep 1, 2022.
- Ghana Statistical Service, Ghana Health Service. Measure DHS: Ghana: demographic and health survey 2014. 2015.
- Ghana Health Service FHD. Family health division annual report 2016. 2017.
- Ghana GSS. Population and housing census: national analytical report. Accra-Ghana Ghana Stat Serv. 2010;2013.
- Snedecor GW, Cochran WG. Statistical methods, 8th Ed. Ames Iowa State Univ Press Iowa. 1989;54:71-82.
- Anlaakuu P, Anto F. Anaemia in pregnancy and associated factors: a cross sectional study of antenatal attendants at the Sunyani Municipal Hospital, Ghana. BMC research notes. 2017 Aug 11;10(1):402. PubMed | Google Scholar
- Padonou G, Le Port A, Cottrell G, Guerra J, Choudat I, Rachas A et al. Prematurity, intrauterine growth retardation and low birth weight: risk factors in a malaria-endemic area in southern Benin. Trans R Soc Trop Med Hyg. 2014;108(2):77-83. PubMed | Google Scholar
- Mikomangwa WP, Minzi OMS, Aklillu E, Kamuhabwa AAR. Adverse birth outcomes among mothers who received intermittent preventive treatment with Sulphadoxine-Pyrimethamine in the low malaria transmission region. BMC Pregnancy Childbirth. 2019;19(1):1-11. PubMed | Google Scholar
- Alanazi NH, Bohassan EAA, Alsabi AF, Bohassan RHA, Alenazi AHN. Iron deficiency anemia with pica syndrome in 5 years old child. Egypt J Hosp Med. 2018;73(4):6577-80. Google Scholar
- Kettaneh A, Eclache V, Fain O, Sontag C, Uzan M, Carbillon L et al. Pica and food craving in patients with iron-deficiency anemia: a case-control study in France. Am J Med. 2005;118(2):185-8. PubMed | Google Scholar
- Orish VN, Onyeabor OS, Boampong JN, Acquah S, Sanyaolu AO, Iriemenam NC. The effects of malaria and HIV co-infection on hemoglobin levels among pregnant women in Sekondi-Takoradi, Ghana. Int J Gynecol Obstet. 2013;120(3):236-9. PubMed | Google Scholar
- Wemakor A. Prevalence and determinants of anaemia in pregnant women receiving antenatal care at a tertiary referral hospital in Northern Ghana. BMC Pregnancy Childbirth. 2019;19(1):1-11. PubMed | Google Scholar
- Ayensu J, Annan R, Lutterodt H, Edusei A, Peng LS. Prevalence of anaemia and low intake of dietary nutrients in pregnant women living in rural and urban areas in the Ashanti region of Ghana. PLoS One. 2020;15(1):e0226026. PubMed | Google Scholar
- Glover-Amengor M, Owusu WB, Akanmori BD. Determinants of anaemia in pregnancy in Sekyere West District, Ghana. Ghana Med J. 2005;39(3):102. PubMed | Google Scholar
- Saaka M, Oladele J, Larbi A, Hoeschle-Zeledon I. Dietary diversity is not associated with haematological status of pregnant women resident in rural areas of northern Ghana. J Nutr Metab. 2017;2017:8497892. PubMed | Google Scholar
- Olatunbosun OA, Abasiattai AM, Bassey EA, James RS, Ibanga G, Morgan A. Prevalence of anemia among pregnant women at booking in the University of Uyo Teaching Hospital, Uyo, Nigeria. Biomed Res Int. 2014;2014:849080. PubMed | Google Scholar
- Tunkyi K, Moodley J. Prevalence of anaemia in pregnancy in a regional health facility in South Africa. South African Med J. 2016;106(1):101-4. PubMed | Google Scholar
- Rezk M, Marawan H, Dawood R, Masood A, Abo-Elnasr M. Prevalence and risk factors of iron-deficiency anaemia among pregnant women in rural districts of Menoufia governorate, Egypt. J Obstet Gynaecol (Lahore). 2015;35(7):663-6. PubMed | Google Scholar
- Okube OT, Mirie W, Odhiambo E, Sabina W, Habtu M. Prevalence and factors associated with anaemia among pregnant women attending antenatal clinic in the second and third trimesters at pumwani maternity hospital, Kenya. MKU Rwanda. 2016. Google Scholar
- Ullah A, Sohaib M, Saeed F, Iqbal S. Prevalence of anemia and associated risk factors among pregnant women in Lahore, Pakistan. Women Health. 2019;59(6):660-71. PubMed | Google Scholar
- Siddiqui MZ, Goli S, Reja T, Doshi R, Chakravorty S, Tiwari C et al. Prevalence of anemia and its determinants among pregnant, lactating, and nonpregnant nonlactating women in India. SAGE Open. 2017;7(3):1-10. Google Scholar
- Jufar AH, Zewde T. Prevalence of anemia among pregnant women attending antenatal care at tikur anbessa specialized hospital, Addis Ababa Ethiopia. J Hematol Thromb Dis. 2014;2(125):2. Google Scholar
- Lebso M, Anato A, Loha E. Prevalence of anemia and associated factors among pregnant women in Southern Ethiopia: a community based cross-sectional study. PLoS One. 2017;12(12):1-11. PubMed | Google Scholar
- Obai G, Odongo P, Wanyama R. Prevalence of anaemia and associated risk factors among pregnant women attending antenatal care in Gulu and Hoima Regional Hospitals in Uganda: a cross sectional study. BMC Pregnancy Childbirth. 2016 Apr 11;16:76. PubMed | Google Scholar
- Tadesse SE, Seid O, Mariam YG, Fekadu A, Wasihun Y, Endris K et al. Determinants of anemia among pregnant mothers attending antenatal care in Dessie town health facilities, northern central Ethiopia, unmatched case -control study. PLoS One. 2017 Mar 13;12(3):e0173173. PubMed | Google Scholar
- Saini RK, Nile SH, Keum YS. Food science and technology for management of iron deficiency in humans: a review. Trends Food Sci Technol. 2016;53:13-22. Google Scholar
- Karaoglu L, Pehlivan E, Egri M, Deprem C, Gunes G, Genc MF et al. The prevalence of nutritional anemia in pregnancy in an east Anatolian province, Turkey. BMC Public Health. 2010 Jun 10;10:329. PubMed | Google Scholar
- Stephen G, Mgongo M, Hussein Hashim T, Katanga J, Stray-Pedersen B, Msuya SE. Anaemia in pregnancy: prevalence, risk factors, and adverse perinatal outcomes in Northern Tanzania. Anemia. 2018 May 2;2018:1846280. PubMed | Google Scholar
- Bodeau-livinec F, Briand V, Berger J, Xiong X, Massougbodji A, Day KP et al. Maternal anemia in Benin: prevalence, risk factors, and association with low birth weight. Am J Trop Med Hyg. 2011 Sep;85(3):414-20. PubMed | Google Scholar
- Alemu T, Umeta M. Reproductive and obstetric factors are key predictors of maternal anemia during pregnancy in Ethiopia: evidence from demographic and health survey (2011). Anemia. 2015;2015:649815. PubMed | Google Scholar
- Kassa GM, Muche AA, Berhe AK, Fekadu GA. Prevalence and determinants of anemia among pregnant women in Ethiopia; a systematic review and meta-analysis. BMC Hematol. 2017 Dec 17;17(1):17. PubMed | Google Scholar
- Kefiyalew F, Zemene E, Asres Y, Gedefaw L. Anemia among pregnant women in Southeast Ethiopia: prevalence, severity and associated risk factors Methods Results. BMC Res Notes. 2014 Nov 3;7:771. PubMed | Google Scholar




