Tuberculosis suspicion in Chadian Health Centers: evaluation of the management system and zoonotic aspect of the disease
Richard Bongo Nare Ngandolo, Lamireou Didi, Diguimbaye-Djaïbé Colette, Taddio Sylvain, Addo Kennedy Kwasi, Kazwala Rudovick Ruben, Oumar Abdelhadi, Daugla Doumagoum Moto, Alambédji Rianatou, Koné Phillip, Jakob Zinsstag, Bassirou Bonfoh
Corresponding author: Richard Bongo Nare Ngandolo, Institut de Recherche en Élevage pour le Développement (IRED), BP 433 N´Djamena, Tchad 
Received: 15 Sep 2020 - Accepted: 24 Oct 2020 - Published: 14 May 2021
Domain: Infectious diseases epidemiology
Keywords: Chad, human tuberculosis, Mycobacterium bovis, Mycobacterium avium, zoonosis
©Richard Bongo Nare Ngandolo et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Richard Bongo Nare Ngandolo et al. Tuberculosis suspicion in Chadian Health Centers: evaluation of the management system and zoonotic aspect of the disease. PAMJ-One Health. 2021;5:6. [doi: 10.11604/pamj-oh.2021.5.6.25872]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/5/6/full
Research 
Tuberculosis suspicion in Chadian Health Centers: evaluation of the management system and zoonotic aspect of the disease
Tuberculosis suspicion in Chadian Health Centers: evaluation of the management system and zoonotic aspect of the disease
![]() Richard Bongo Nare Ngandolo1,&, Lamireou Didi1, Diguimbaye-Djaïbé Colette1, Taddio Sylvain1, Addo Kennedy Kwasi2, Kazwala Rudovick Ruben3, Oumar Abdelhadi4, Daugla Doumagoum Moto5, Alambédji Rianatou6, Koné Phillip6, Jakob Zinsstag7, Bassirou Bonfoh8
Richard Bongo Nare Ngandolo1,&, Lamireou Didi1, Diguimbaye-Djaïbé Colette1, Taddio Sylvain1, Addo Kennedy Kwasi2, Kazwala Rudovick Ruben3, Oumar Abdelhadi4, Daugla Doumagoum Moto5, Alambédji Rianatou6, Koné Phillip6, Jakob Zinsstag7, Bassirou Bonfoh8
&Corresponding author
Introduction: in 2016, 1.7 million of people died from tuberculosis caused by bacteria of the Mycobacterium tuberculosis complex, of which some may have a zoonotic origin. The mycobacterial disease endemicity is worrying in Africa owing to the absence of a reliable strategy in relation with disease ecology. The objective of the study is to assess the reliability of different components of the tuberculosis research and surveillance system in Chad.
Methods: in 2012, a cross-sectional study was conducted in order to assess the importance of tuberculosis in southern Chad and Lake Chad regions. Nine health centers located in areas with a high proportion of pastoralist populations were visited. Microbiological and molecular diagnoses were performed on samples collected from tuberculosis-suspected patients.
Results: we assessed one quarter (26.4%) of all the suspected cases in our study site, as estimated in 2012. More males were infected than females (329 versus 147) and most of the suspected cases were aged between 16 and 60 years. Of the 478 sputa collected, 77 were identified as infected with acid fast bacilli, from which 71 were of the genus Mycobacterium, including 45 Mycobacterium tuberculosis, a case of co-infection Mycobacterium bovis/Mycobacterium avium, and 25 non-tuberculosis mycobacteria. Twelve suspected cases harbored other mycobacteria.
Conclusion: the co-infection Mycobacterium bovis/Mycobacterium avium found in a poultry farmer was the first such co-infection in Chad related to human tuberculosis. Indeed, this finding highlights the importance of the zoonotic aspect of mycobacterial diseases and proves the value of molecular characterization in the tuberculosis surveillance.
Tuberculosis (TB) is caused by bacteria of the Mycobacterium tuberculosis complex (MTC) which includes M. tuberculosis, M. bovis, M. africanum and other mycobacterial species and represents a major health issue [1]. In 2016, an estimated 10.4 million people were infected with MTC bacteria and an estimated 1.7 million died from TB [2]. In addition, non-tuberculosis mycobacteria (NTM) cause non-TB infections, posing a significant risk in sub-Saharan Africa owing to the absence of diagnostic and treatment guidelines. M. avium is responsible for most cases of NTM lung infections worldwide [3,4]. The incidence of disseminated NTM, most notably the M. avium complex (MAC), has been, however, decreasing in the last 10 years due to potent anti-HIV treatment and efficient prophylaxis [5]. Little is currently known about this diverse group of bacteria [6].
Many surveillance systems of infectious diseases in human populations in Africa ignore the impact of an ecosystem on the outbreak, the propagation or the endemicity of diseases. Most surveillance strategies do not take into account the importance of a multidisciplinary research approach when seeking to identify infection pathways and develop diagnostic tools. In addition, most funding and research concerning mycobacterial diseases in Africa today focuses on determining disease prevalence and incidence in a given area without considering the development of epidemiological frameworks and diagnostic tools in order to establish feasible surveillance systems. Mycobacterial management remains complex on the continent and most national infectious disease control programmes are facing technical problems such as the lack of adequate infrastructure, equipment and well trained scientific staff, even when written surveillance strategy documents and an allocated budget are available. The main goal of most African national TB programmes is to organize disease surveillance, support specific TB research, to measure the progress of activities related to the TB surveillance, identify communities at risk and to scout out new models of intervention. In order to achieve these objectives, promoting TB research is the single most important action of African national TB programmes.
One of the first components to establish research capacity within a TB programme is the setting up of a reference laboratory capable to isolate MTC strains, to characterize different Mycobacteria species and to perform reliable multi drug resistant diagnostic. Even with new methods such GenExpert being nowadays available for rapid and accurate diagnostic of TB, their maintenance and accuracy in term of MDR diagnosis has yet to be validated for African settings. Thus, culture of mycobacterial strains will remain the gold standard in TB diagnosis for a long time in African countries. The national TB programme in Chad, which is on the way to establish its reference culture laboratory, has come together with the Institut de Recherche en Élevage pour le Développement (IRED) that has already established a diagnostic platform involving both culture and molecular typing of mycobacterial strains. In the frame of this collaboration, assessing the reliability of different components of the TB research system was one of the strategies adopted by these two national institutions before the establishment of the surveillance of the anti-TB drug resistance at the national level [7]. This evaluation process involved both field and laboratory activities in order to establish and align Good Clinical Practices (GCP) and Good Clinical and Laboratory Practices (GCLP) across these two institutions. Our study, based on the One Health concept, was undertaken by both medical and veterinarian staff with the objectives to identify the main causes of the weakness of the Chadian TB surveillance mechanism, to describe the implication of zoonotic TB in the incidence of disease and to characterize the epidemiological profile of the disease in Chad.
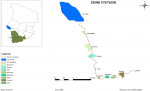
A cross sectional study was conducted from June to September 2012. During four month, nine health centers located in the Lake Chad Islands and the southern Chad regions with high pastoralist activities were visited for data collection (Figure 1). In order to actively coordinate data and sample collection, a network was established involving partners from the Ministry of Public Health and the Ministry of Pastoral Development and Animal Production. These two ministries are involved the national TB programme that is represented in the field by the nine health centers and IRED, represented by the veterinarian mycobacterial laboratory staff. Two other partners representing local communities were also included in the data collection process: the Association of Motorcycle Taxi and two inter-urban transport agencies (Sud Voyage and Société Tchadienne de Transport et de Location (STTL)). Motorcycle taxis were used to transport data and collected samples from health centers to inter-urban transport agencies and then to the mycobacterial laboratory in IRED. However, inter-urban transport agencies were in charge of transporting both collected data and samples from the field to N´Djamena. These partners were trained at the beginning of the study on basic knowledge related to GCP and GCLP concerning collection and transport of infectious material according to class 6.1 as described by the International Air Transport Association (IATA). Mobile phones were used for maintaining permanent contact between field and coordination staff at IRED in N´Djamena as part of the sample tracking system. Our study was approved by the coordinating board of the national TB control programme.
Patients: during our study, clinical samples were collected from 478 suspected TB patients present in nine health centers at Sarh, Koumra, Doba, Bébidja, Moundou, Kélo, Bongor, Guelendeng (Mogrom) and Lake Chad region (Figure 1). These patients were enrolled in the study after clinical examination done by the physician in charge of TB infections and/or lung diseases in each health center. All patients presenting extra pulmonary TB clinical signs or having suffered from cough during the two last weeks prior to the hospital visit were enrolled in the data collection process. A volume of 5 ml was taken from each clinical sample (sputum), collected for the purpose of microscopic diagnosis at the health center, and added to 5 ml of cetyl pyridinum chlorid (CPC 5%) in a 50 ml falcon tube. Specimens were kept at room temperature for at least 5 days and then sent to IRED for bacteriological and molecular diagnosis.
Sample processing: for the bacteriological diagnosis, clinical specimens were processed as described previously by Diguimbaye-Djaibé and colleagues [8] in a bio safety laboratory (BSL) 2 laboratory. The volume of each specimen collected in the falcon tube was first adjusted to 10 ml by removing a small quantity or by adding sterile distilled water. The whole content was vortexed and washed with 30 ml of phosphate buffer pH 6.8 followed by centrifugation at 3500 rpm for 15 min. The supernatant was subsequently transferred into a cup containing chloride solution (5%), and the sediment was homogenized with 2 ml of sterile distilled water. A volume of 0.5 ml of each homogenized specimen was mixed with 8 ml of modified MGIT medium (MGIT for mycobacteria growth indicator tube), containing oleic Acid-Albumin-Dextrose-Catalase (OADC), lot N°245116 and PANTA (for polymyxin B, amphotericin B, nalidixic acid, trimethoprim, azlocillin), becton Dickinson and Campany (BD) lot N°245114. Cultures were incubated at 37°C (without CO2) and checked once a week until a medium turbidity was observed until up to eight weeks. Presence of Acid Fast Bacilli (AFB) in turbid cultures was confirmed by smear stained using the Ziehl Neelsen method. A sample of 0.5 ml from each non-contaminated AFB positive culture was centrifuged at maximum speed (3000 rpm) for 5 min, the supernatant was removed and the pellet re-suspended in 0.5 ml of sterile distilled water. The AFB mixtures were heat killed at 85°C for 15 min in a heat block in order to extract the mycobacterial Deoxyribonucleic Acid (DNA), and these specimens were stored at -20°C prior to molecular diagnosis.
One hundred molecular typing of harvested strains was performed using firstly the Polymerase Chain Reaction (PCR) genus typing method for purpose of mycobacterium genus identification, then for Mycobacterium tuberculosis complex (MTC) group identification [9]. The genus typing PCR protocol was used to identify species from the Mycobacterium genus, but also to differentiate species of the MTC from M. avium, M. intracellulare and other Mycobacterium species. Six different primers were used for this purpose. Firstly, a sequence region within 16S rRNA gene specific for the Mycobacterium genus was targeted. The two primers MYCGEN-F and MYCGEN-R are designed to amplify a specific PCR product from genomic DNA of all known mycobacteria. Secondly, the PCR mix also includes primers that are specific for a hyper variable region of the 16S rRNA gene of M. intracellulare (MYCINT-F) and M. avium (MYCAV-R), respectively, giving one additional PCR product if the DNA product is any of these two species. Thirdly, species from the MTC can also be identified due to two primers (TB-F, TB-R) that target the MPB70 gene, specific for mycobacteria from the complex. The following primers were used: 100 μM MYCGEN-F 5´ - AGA GTT TGA TCC TGG CTC AG - 3´; 100 μM MYCGEN-R 5´ - TGC ACA CAG GCC ACA AGG GA - 3´; 100 μM MYCAV-R 5´ - ACC AGA AGA CAT GCG TCT TG - 3´; 100 μM MYCINT- F 5´ - CCT TTA GGC GCA TGT CTT TA - 3´; 100 μM TB1-F 5´ - GAA CAA TCC GGA GTT GAC AA - 3´; 100 μM TB1-R 5´ - AGC ACG CTG TCA ATC ATG TA - 3´;
The volume of one reaction included 6.2 μl of purified and manufactured Qiagen DNA/RNA free water (Cat N° 129114 and Lot N° 142315905) and 10 μl of Master mixt (lot n°203446 which include DNA polymerase, buffer, MgCl2, and dNTPs); in which 0.3 μl of each primer described above was added. The following PCR programme was applied: first denaturation: 95°C for 10 min, {denaturation: 95°C for 1 min, elongation 61°C for 0.5 min, annealing 72°C for 2 min} 35 cycles; final elongation: 72°C 10 min. Hold: 4°C + ∞.
The following primers were used: 100 μM RD4-FlankFW 5´ - CTC GTC GAA GGC CAC TAA AG - 3´; 100 μM RD4-FlankRev 5´ - AAG GCG AAC AGA TTC AGC AT - 3´; 100 μM RD4-InternalFW 5´ - ACA CGC TGG CGA AGT ATA GC - 3´; 100 μM RD9-FlankFW 5´ - AAC ACG GTC ACG TTG TCG TG - 3´; 100 μM RD9-FlankRev 5´ - CAA ACC AGC AGC TGT CGT TG - 3´; 100 μM RD9-InternalRev 5´ - TTG CTT CCC CGG TTC GTC TG - 3´; For the second PCR protocol [10], the volume of one reaction included 7.1 μl of purified and manufactured Qiagen DNA/RNA free water (cat n° 129114 and lot n° 142315905) and 10 μl of master mix (lot n°203445 which include DNA polymerase, buffer, MgCl2, and dNTPs); in which 0.3 μl of each primer described above was added. The following PCR programme was applied: first denaturation: 95°C for 15 min, {denaturation: 95°C for 1 min, elongation 55°C for 0.5 min, annealing 72°C for 1 min} 35 cycles. Final elongation: 72°C for 10 min. Hold: 4°C + ∞. Data entry was done using the Microsoft ACCESS and the analysis was done using STATA13 software (Stata®). Multivariate analysis was performed in order to identify the main risk factor, the most related to the diseases.
A total of 478 patients were recruited from nine health centers during a period of 4 month with suspected pulmonary TB cases. Sputum specimens were collected from each patient. Most suspected cases were recruited in Kélo (N=164) and two other neighboring towns, namely Moundou (N=78) and Bongor (N=55) (Table 1), followed by the Lake Chad region where 73 patients were registered. Lowest numbers were recruited in Doba, Sarh and Guelendeng with 8, 11 and 19 patients, respectively. The largest proportion of the collected samples was sent by the staff from Kélo (75.57%), Bébidja (50.74%), Doba (47.05%), Guéléndeng (46.34%), and Koumra (43.90%) (Table 1). More male patients were recorded than females (329 versus 147), and most of the suspected cases were aged between 16 and 60 years (Table 2). Considering the occupational status, TB suspected patients were consistently represented within workmen (28.87%), cultivators (23.64%) and housewives (20.50%), followed by students (9.83%), people without occupation (6.90%), official workers (5.64%) and butchers (1.46%) (Table 2).
Field results were confirmed in the mycobacterial laboratory by isolating the different types of bacterial strains from 83 TB suspected patients. The distribution of the strains according to age group is presented in Table 3. Bacteria that belong to the MTC group represented 55% (46/83) of all bacterial analyzed, composed of mostly M. tuberculosis (45/83) and one M. bovis case (1/83). Surprisingly, one case of M. bovis/M. avium co-infection was identified in a specimen harvested from a poultry farmer living in Bébidja. NTM were present in more than 30% of isolated bacteria (25/83). Other bacteria than mycobacteria were also characterized, representing 14% (12/83). The 83 patients from whom bacteria strains were isolated represented only 17.4% of all suspected TB cases. However, 75% (62/83) of these bacteria were isolated from males and were harvested from sputum collected from patients aged 16 to 60 years (78/83). Similar demographic conditions applied when assessing MTC group bacteria; most of mycobacteria from this complex were isolated from males (36/83) and patients aged 16 to 60 years (43/83), including the case of M. bovis/M. avium co-infection. However, multivariate analysis performed with mycobacteria infection or MTC infection as disease (outcomes variable) revealed that in both situations, the explanatory variable “sex” was the main risk factor for disease. According to the odds ratio (OR) women were at 3.3 times higher risk than men (P < 0.05, OR=3.30 95%: 1.07-10.33). However, management and follow-up of the patients depend only on the material, financial and human resources sent by the Tuberculosis National Program or Ministry of Public Health in each health center.
In 2012, the total population at our study site was estimated to be 2,137,838 people among whom 6882 were suspected of TB infection during a time period of 4 months [11]. The suspected cases assessed within our study represented approximately one third of all those suspected cases in 2012. This was representative because our study period was conducted between 1st June to 30th of September and it represented only one third (four month) of the whole year. Most of all TB deaths that occur in low- and middle-income countries were reported among women at childbearing age in 2018 [12] which is in line with our study identifying women from this age group being at highest risk.
The high incidence of bovine TB and its associated mortality in Africa underlines the emergency of investigating the importance and implication of zoonotic TB in the continent [13]. The sub-optimal TB management in Africa is due to the weakness of the surveillance systems. Even when suspected cases are reported and treated, failure of TB treatments and cases of TB patients that evade the follow up process are commonly reported [14]. In addition, even when the Directly Observed Treatment Short-course (DOTS) strategy is applied, in most African health centers located in the sub-Sahara region, relatives are in charge of assisting a patient retained to a referral center for long-term treatment. In this scenario, the assistance is often given by women and usually without knowledge on the infectious aspects of the disease. Instructions related to TB infectious features are commonly passed to the patients´ relatives by the health center staff, as preventive measure. However, most new cases are detected at the infectious stage of the disease after these patients may have already significantly exposed their family before their first visit at the health center. Thus, the situation is quite dramatic in Africa where most of the national health systems are focusing on the curative aspects of the infectious diseases rather than preventing people from getting infected. Indeed, in 2016, the sub-Saharan Africa region presented the highest proportion of new cases per population in the world with about 100 to up to 300 cases per 100,000 populations [12]. This failure to decrease TB numbers is being observed despite the creation of African national TB programmes between 1980 and 1990 with the main objective to eradicate the disease from the continent. However, their main activities were focused on the identification of new cases and the treatment of infected persons rather than looking at the causes of disease endemicity [15]. In Chad, the situation remained unchanged from 2009 to 2012 (151 per 100,000 people) [16].
One of the main surveillance system components that could contribute to the identification of the causes of treatment failure is the existence of a laboratory with a well-established data collection mechanism and the diagnostic capacity to isolate and characterize TB causative agents. The limited quantity of suspected cases enrolled in our study (25%) suggests that the passive data collection system is a major weakness of our surveillance mechanism. Even by involving communities and contracting private services for sample routing, gaps persist at the health center level. The Chadian health system is organized according to central, intermediate and peripheral health system levels [11]. Health centers are part of the intermediate and peripheral levels, classified as regional hospitals, district hospitals and responsibility areas. During the data collection period, samples came from four district hospitals (Kélo, Bébidja, Guélendeng and Lake Chad) and five regional hospitals (Doba, Koumara, Bongor, Moundou and Sarh). Data sent by district hospitals were representative compare to those sent by regional hospitals suggesting a specific need of district hospitals in terms of TB diagnostic. In fact, most of the regional hospitals possess laboratories and qualified staff that are able to conduct the Ziehl Neelsen staining method and to identify acid fast bacilli using microscopy compare with district hospitals. However, in terms of TB surveillance, quality control of the microscopy result is an important process for auditing technicians in a quality control network and both central and peripheral (districts and health centers) levels must be involved in the auditing process through a well-established diagnostic mechanism. Thus, the TB diagnostic tools and qualified personnel have to be implemented at each level of the surveillance mechanism chain. As shown previously [17], low rates of TB patient detection in health centers in Africa is due to inadequate health coverage and poor development of the laboratory network. Sub-detection is compounded by several factors, such as the verticality of national TB programmes which are managed centrally but are less supported at the intermediate and peripheral level and their frequent reliance on external aid imposing their methods [18]. Low motivation of the staff at the intermediate and peripheral levels is an aspect that needs to be addressed in addition [19].
Most African health systems define new TB cases following the WHO criteria [20]. The base of pulmonary TB suspicion in African health centers are coughing during two weeks period, in relation with weight loss, coughing with blood or mucus, weakness or fatigue, fever and chills, night sweats, and lack of appetite. During our data and samples collection period, all 478 specimens were collected according to these criteria and they were all from patients suspected as pulmonary TB cases by a physician employed for this purpose. These data (Table 1) highlighted in general the importance of the lung infections in Chad and demonstrated also the need of a strong collaboration between the sites clinical staff coordinated by the National TB Programme and medical research institution in order to strengthen identification process of drug resistant strains cases, when the issue is addressed to TB monitoring. The experiences acquired from DOTS strategy helped in 2002 to broaden the outlook of the initial strategies into three additional points among which the issue of improving epidemiological surveillance of tuberculosis through the development of intermediate level to oversee the activities of district hospitals and laboratory network. The laboratory network should include culture laboratories at the intermediate level and a reference laboratory. This latter should be responsible for achieving the sensitivity tests, trainings, quality control, supervision and research activities [17]. This present research activities done in collaboration with the Chadian National program of TB allowed us to improve our knowledge on the epidemiological situation in rural setting.
By socio professional occupation, the suspected lung infections seemed to be more frequent among workmen, cultivators and housewives. However, after bacteriological diagnostic, 83 patients were identified as infected by different types of bacteria strains and suspected cases due to mycobacterial infection in general represented 85.50%, then new cases of TB infection confirmed by molecular diagnostic tools represented more than 50% of these bacterial infections. They were more frequent among patients aged from 16 to 60 years specially cultivators, workmen and then housewives as shown by previous studies done in Zimbabwe, Kenya and Malawi that confirmed that tuberculosis cases in primary health and chest clinic settings, were in 40 to 70% among adults with cough for 3 weeks or longer [18,21]. Outcomes from our study proved that most of the isolated NTM were from patients aged from 16 to 45 years old (19/24) and work females (6/24) as shown by E. Braun et al. 2012, [5] that pulmonary diseases caused by NTM is commonly encountered in middle-aged men with chronic lung disease and elderly female patients with no-preexisting lung disease. Isolation of NTM strains from TB suspected patients could lead to a TB treatment failure because in countries with high rate of TB and lack of accurate laboratory procedures, patients with positive AFB and pathological radiographic finding are erroneously treated with anti-tuberculosis drugs [22]. Indeed, this group of mycobacteria is commonly considered to be clinically less important than M. tuberculosis and was note advised to be reported to public health staff, but the identified species are being considered as causative agents of human disease with increasing frequency [4]. Transmission of these bacteria to humans is seemed to be directly from environment sources [23] and the isolation of a M. avium strain from a poultry farmer during our study supports this hypothesis. This hypothesis supports the fact that the infection source of lung disease due to M. avium is feaces excreted by infected birds in the environment [24]. Pulmonary disease due to mycobacteria in bird is most associated with M. avium complex [3] and the fact that this poultry farmer presented a case of co-infection M. bovis/M. avium supports this second hypothesis.
M. avium complex (MAC) infection is usually described as poultry and swine disease, and the pathogen is commonly isolated from faeces samples collected from birds, but not swines [25]. The first human pulmonary disease due to MAC infection has been described in males from 45 to 65 years old with [3]. Isolation of organism is the main way to diagnose disease caused by MAC. M. tuberculosis, M. bovis and MAC bacteria are all together in microscope described as AFB. However, culture and identification by DNA probes are required to specify these species [3]. As highlighted by DA Ashford et al. (2001) [24], species characterization of MAC requires expertise that is rare in the developing world. Nowadays, in some African countries, these scientific methods were established and could be used in terms of regional network in order to improve the TB surveillance mechanism in the continent. During our study, culture and molecular diagnostic were used at the internal level for this purpose and a case of co-infection M. bovis/M. avium was identified. This finding highlighted the implication of zoonotic TB among people with permanent contact with animals. The disease is rare in individuals not affected by Acquired Immunodeficiency Syndrome (AIDS) [26]. Our hypothesis is that, the poultry farmer maybe was firstly infected by M. bovis, and then due to the weakness of his immune system, he had secondly contracted the disease caused by M. avium. In South Africa, M. avium was isolated from patients suffering from AIDS and with CD4 cell count less than 100 cells/mm3, then the point prevalence of disseminated MAC infection was 10%, compare with other African studies which reported that the infection is uncommon [27]. This situation should be due to the lack of culture and molecular diagnostic tools. However, in Nigeria in 2006 [28] of 65 mycobacterial isolates submitted to the tests, 15 were characterized as environmental mycobacteria among which 9 (20.69%) were descried as M. avium. In a cross sectional studies involving HIV-1-infected patients, the prevalence of MAC bacteremia ranged from 0% to 6% of hospitalized patients in Zambia, Kenya, Malawi, Tanzania and Uganda [29].
A case of granuloma co-infection with M. bovis and MAC was already identified in red deer in Portugal [30]. However, cases of co-infection in humans are uncommon. Our finding should be one of the first cases of co-infection M. bovis/M. avium identified in Africa. It was demonstrated that in vitro susceptibility testing of M. tuberculosis has been well validated to correlate with clinical response but not for NTM species. This situation could be one of the causes of failing in TB suspected patients treatment. Outcomes from the present study shows that 5% (25/478) of the suspected TB patient were Positive Non Tuberculosis Mycobacteria (PNTM) cases and they could be erroneously treated with anti-tuberculosis drugs. Such treatment could fail and interpreted as cases of drug resistant because the administered molecules were not adequate. Identifying such cases as part of TB mono or multi drug resistance monitoring, will help to surely manage the real TB drug resistant cases and save time in the treatment regimen.
Indeed, our surveillance mechanism could reach the whole TB suspected cases in our study site if our data collection period was extended to the whole year 2012 and by involving new partners such as communities and private inter urban transport agencies in the data collection process that helped to transport the collected samples in best conditions. However, we identified some gaps for instance on the health center level within the national health system, motivation of the staff in charge of data collection, the quality of the transport media, and the transport times threshold that have to be discussed. Otherwise, the co-infection M. bovis/M. avium found in a poultry farmer was the first such co-infection in Chad related to human TB. This finding highlights the importance of the zoonotic aspect of mycobacterial diseases and proves the value of molecular characterization in the tuberculosis surveillance.
What is known about this topic
- In Chad, tuberculosis caused by M. tuberculosis and M. bovis was respectively described in human and animal populations. It also proven that the molecular characterizations of the first 40 M. tuberculosis isolates from Chad revealed a high proportion of isolates of the Cameroon family (33%), of which one isolate showed a monodrug resistance to isoniazid. Concerning M. bovis strains isolated form slaughtered animals, spoligotyping shows a homogenetic population structure and lack of spacer 30, as were found in neighboring Cameroon and Nigeria suggesting a transborder and ongoing transmission between cattle.
What this study adds
- This study highlights the importance of the zoonotic aspect of mycobacterial diseases and proves the value of molecular characterization in the tuberculosis surveillance in order to trace back the disease origin.
The authors declare no competing interest.
Richard Bongo Nare Ngandolo, Lamireou Didi and Diguimbaye-Djaïbé Colette initiated the idea of the paper and wrote the first draft. Addo Kennedy Kwasi, Kazwala Rudovick Ruben, Moto, Alambédji Rianatou, Koné Phillip, Jakob Zinsstag, Bassirou Bonfoh did the data analysis and organize the outcome exploitation. All other author helped with data collection and laboratory works then participate in writing the manuscript. All the authors have read and agreed to the final manuscript.
This work was supported through the DELTAS Africa Initiative (Afrique One-ASPIRE/DEL-15-008). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)´s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa´s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107753/A/15/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust or the UK government. We also wish to thank our collaborators from different health centers, inter urban transport agencies (STTL and Sud voyage) and the communities for their commitment and availability during the data collect period. We also thank Jasmina Saric for reviewing the manuscript.
Table 1: summary of field activities in 2012: collected data compare to those from the Ministry of Public Health
Table 2: pulmonary TB suspected cases in health centers, distribution by age group, sex and socio professional occupations
Table 3: bacterial strains isolated from suspected patients, distribution by sex and age groups
Figure 1: study sites
- Kaneene JB, Thoen CO. Tuberculosis. J Am Vet Med Assoc. 2004;224(5):685-691. PubMed | Google Scholar
- World Health Organisation. Fact sheets on tuberculosis. WHO. 2018. Accessed 24 November 2020.
- Glassroth J. Pulmonary disease due to nontuberculous mycobacteria. Chest. 2008;133(1):243-251. PubMed | Google Scholar
- Thomson RM, Yew WW. When and how to treat pulmonary non-tuberculous mycobacterial diseases. Respirology. 2009;14(1):12-26. PubMed | Google Scholar
- Braun E, Sprecher H, Davidson S, Kassis I. Epidemiology and clinical significance of non-tuberculous mycobacteria isolated from pulmonary specimens. Int J Tuberc Lung Dis. 2013 Jan;17(1):96-9. PubMed | Google Scholar
- Wilson ML. Reducing the global burden of mycobacterial infections one more piece of the puzzle. Am J Clin Pathol. 2008;130(6):849-852. PubMed | Google Scholar
- World Health Organisation. Guideline for surveillance of drug resistance in tuberculosis. 4th ed Geneva, Switzerland. 2009. Google Scholar
- Diguimbaye C, Hilty M, Ngandolo R, Mahamat HH, Pfyffer GE, Baggi F et al. Molecular characterization and drug resistance testing of mycobacterium tuberculosis Isolates from Chad. J Clin Microbiol. 2006;44(4):1575-1577. PubMed | Google Scholar
- Berg S, Berg Stephan. Standard operating procedure for mycobacterium genus typing. Protocol Collected and Assembled from References. 2008.
- Berg Stephan. Satandard operating procedure for deletion typing - a method for differentiation of mycobacterium tuberculosis complex. Information on Deletion Typing. 2008.
- ReliefWeb. Tchad: Annuaire des statistiques sanitaires - Tome A 31e édition année 2017 - Chad. Accessed November 24, 2020.
- World Health Organization. Global Tuberculosis Report. Genva Switzerland. 2017.
- Nastasee S. Zoonotic tuberculosis in Africa. Med J Ther. 2008;2(1):53-58.
- Programme National de Lutte contre la Tuberculose. Guide technique. 3rd ed N´Djamena, Tchad. 2010.
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet Lond Engl. 2006;367(9514):926-937. PubMed | Google Scholar
- The World Bank. Incidence of tuberculosis (per 100,000 people). Accessed 22 November 2020.
- Calameo La tuberculose en Afrique: épidémiologie et mesures de lutte. Accessed 16 November 2020.
- Van Cleeff MRA, Kivihya-Ndugga L, Githui W, Nganga L, Odhiambo J, Klatser PR. A comprehensive study of the efficiency of the routine pulmonary tuberculosis diagnostic process in Nairobi. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2003;7(2):186-189. PubMed | Google Scholar
- Asif M, Baig MA, Shah MN. Evaluation of the tuberculosis surveillance system in District Hyderabad, Province Sindh-Pakistan, 2012. Int J Trop Dis Health. 2015;1-8. Google Scholar
- WHO. Definitions and reporting framework for tuberculosis. WHO. Accessed 22 November 2020.
- Harries AD, Kamenya A, Subramanyam VR, Maher D, Squire SB, Wirima JJ et al. Screening pulmonary tuberculosis suspects in Malawi: testing different strategies. Trans R Soc Trop Med Hyg. 1997;91(4):416-419. PubMed | Google Scholar
- Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367-416. PubMed | Google Scholar
- Kankya C, Muwonge A, Djønne B, Munyeme M, Opuda-Asibo J, Skjerve E et al. Isolation of non-tuberculous mycobacteria from pastoral ecosystems of Uganda: Public Health significance. BMC Public Health. 2011 May 16;11:320. PubMed | Google Scholar
- Ashford DA, Whitney E, Raghunathan P, Cosivi O. Epidemiology of selected mycobacteria that infect humans and other animals. Rev Sci Tech Int Off Epizoot. 2001;20(1):325-337. PubMed | Google Scholar
- Meissner G, Anz W. Sources of Mycobacterium avium complex infection resulting in human diseases. Am Rev Respir Dis. 1977 Dec;116(6):1057-64. PubMed | Google Scholar
- Horsburgh CRJ. Mycobacterium avium complex infection in the Acquired Immunodeficiency Syndrome. N Engl J Med. 1991 May 9;324(19):1332-8. PubMed | Google Scholar
- Pettipher CA, Karstaedt AS, Hopley M. Prevalence and clinical manifestations of disseminated Mycobacterium avium complex infection in South Africans with acquired immunodeficiency syndrome. Clin Infect Dis Off Publ Infect Dis Soc Am. 2001;33(12):2068-2071. PubMed | Google Scholar
- Mawak J, Gomwalk N, Bello C, Kandakai-Olukemi Y. Human pulmonary infections with bovine and environment (atypical) mycobacteria in Jos, Nigeria. Ghana Med J. 2006;40(4):132-136. PubMed | Google Scholar
- Holmes CB, Losina E, Walensky RP, Yazdanpanah Y, Freedberg KA. Review of human immunodeficiency virus type 1-related opportunistic infections in sub-Saharan Africa. Clin Infect Dis Off Publ Infect Dis Soc Am. 2003;36(5):652-662. PubMed | Google Scholar
- Matos AC, Dias AP, Morais M, Figueira L, Martins MH, Matos M et al. Granuloma coinfection with mycobacterium bovis, mycobacterium avium subsp. paratuberculosis, and corynebacterium pseudotuberculosis in five hunted red deer (cervus elaphus) in Portugal. J Wildl Dis. 2015;51(3):793-794. PubMed | Google Scholar