Risk factors for human anthrax outbreak in Kiruhura District, Southwestern Uganda: a population-based case control study
Richard Migisha, Irene Mbatidde, David Collins Agaba, Eleanor Turyakira, Gabriel Tumwine, Aggrey Byaruhanga, Aggrey Siya, Gad Ndaruhutse Ruzaaza, Halid Kirunda
Corresponding author: Richard Migisha, Department of Physiology, Mbarara University of Science and Technology, Mbarara, Uganda 
Received: 18 Apr 2021 - Accepted: 24 Jun 2021 - Published: 30 Jun 2021
Domain: Epidemiology,Infectious diseases epidemiology
Keywords: Anthrax, risk factors, outbreak, Uganda, attack rate
©Richard Migisha et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Richard Migisha et al. Risk factors for human anthrax outbreak in Kiruhura District, Southwestern Uganda: a population-based case control study. PAMJ-One Health. 2021;5:13. [doi: 10.11604/pamj-oh.2021.5.13.29385]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/5/13/full
Research 
Risk factors for human anthrax outbreak in Kiruhura District, Southwestern Uganda: a population-based case control study
Risk factors for human anthrax outbreak in Kiruhura District, Southwestern Uganda: a population-based case control study
![]() Richard Migisha1,&, Irene Mbatidde2,
Richard Migisha1,&, Irene Mbatidde2, ![]() David Collins Agaba1, Eleanor Turyakira3, Gabriel Tumwine4, Aggrey Byaruhanga5, Aggrey Siya6,
David Collins Agaba1, Eleanor Turyakira3, Gabriel Tumwine4, Aggrey Byaruhanga5, Aggrey Siya6, ![]() Gad Ndaruhutse Ruzaaza3,
Gad Ndaruhutse Ruzaaza3, ![]() Halid Kirunda2
Halid Kirunda2
&Corresponding author
Introduction: in 2018, Uganda experienced recurrent outbreaks of anthrax in both humans and livestock. We aimed to determine risk factors for human anthrax outbreak among residents of Kazo County, Kiruhura District, southwestern Uganda.
Methods: we conducted an unmatched case control study during March-April 2019. We defined a case as having had anthrax infection reported to be diagnosed by a healthcare worker in a resident of Kazo County between May 1st, 2018 and June 1st, 2018. A control was a resident in the nearest neighboring household who had not been diagnosed with anthrax between May 1st and June 1st and who had no symptoms suggestive of anthrax in May 2018. We obtained participants' socio-demographic, clinical and exposure characteristics using a structured questionnaire. We performed logistic regression to identify risk factors forhuman anthrax.
Results: we recruited 101 participants (28 cases and 73 controls) with median age of 34 (IQR; 26-47) years; most (61.4%) were female. The overall attack rate was 1.9%, while the most common clinical manifestations were ulcers (96.4%) and fever (96.4%). The risk factors for contracting human anthrax were: slaughtering of anthrax infected animals (aOR=5.74; 95%CI: 1.39-23.8), consuming of anthrax infected meat (aOR=6.4; 95%CI: 1.53- 6.7) and being male (aOR=12.8; 95%CI: 3.31-49.1).
Conclusion: the point source outbreak in humans was predominantly of the cutaneous form as a result of contact with anthrax infected meat. We recommend community sensitization on safe disposal of carcasses, avoiding slaughtering/handling carcasses, and carrying out routine livestock vaccinations against anthrax in Uganda to avert similar outbreaks in future.
Anthrax is a zoonotic disease caused by Bacillus anthracis, an aerobic, gram-positive and spore-forming bacterium that belongs to the family Bacillaceae [1]. Humans always contract the natural disease directly or indirectly from infected animals or animal products [2,3]. Anthrax in humans is classified into three forms according to clinical features and transmission routes: the cutaneous form - accounting for about 95% of all reported human cases worldwide, the gastrointestinal form, and the pulmonary form [4]. Anthrax is known to cause devastating socio-economic impact in various ways, including animal disease, loss of productivity, loss of income for livestock-dependent populations, and human morbidity and mortality [5]. The disease perpetuates poverty and causes emotional trauma, especially among the poor populations whose livelihoods depend on pastoral farming [6]. In addition, due to destruction of infected animals, household food security is often affected and farmers experience large financial losses [7]. Livestock vaccination in most low income countries is commonly a reactive measure after a major outbreak, rather than being employed as a preventative strategy [8,9]. Moreover, livestock vaccination rates in sub-Saharan Africa are still alarmingly low (0-6%) [10]. In Uganda, anthrax is still a "private good" disease, hence vaccination is only occasionally conducted by farmers as a reaction measure when anthrax outbreaks occur.
Anthrax is among the top seven priority zoonotic diseases in Uganda and neighboring East African countries [11,12]. Uganda is particularly susceptible to zoonotic diseases due to its exceptional biological diversity and escalating population density that brings humans and animals into increasing interaction [13,14]. Furthermore, over 80% of Uganda´s population is involved in agriculture with 58% of which engaged in livestock farming [14]. Several factors including human behavior, poor anthrax surveillance and weak control programs have been implicated in the persistence of anthrax in some countries [15,16]. In addition, most households, and families may consume and sell some of the meat from anthrax infected animals in order to minimize losses associated death of animals that should ideally be safely disposed of [17,18]. This is worsened by the absence of compensation schemes for livestock losses in resource-limited settings. In 2018, recurrent outbreaks of anthrax in both animals and humans were reported in four districts of Uganda, namely: Zombo, Arua, Kween districts in norther Uganda, and Kiruhura district in western Uganda. Regrettably, anthrax outbreaks among humans in Uganda are only minimally documented. In order to generate information to guide future public health interventions, this study was conducted to establish the risk factors associated with contracting human anthrax among residents in affected cattle keeping villages in southwestern Uganda.
Study setting: the study was carried out in Kazo County, Kiruhura District, southwestern Uganda, during March-April 2019. Livestock farming is the main economic activity in the area; it occupies 58% of the households in the district [19]. The total number of households in Kiruhura District is estimated at 67,152, with a total population of about 328,077 persons and household size averaging five persons [20]. Kiruhura District is composed of 562 villages, 91 parishes, 15 sub-counties and two counties [21]. The district annually experiences two rainfall peaks (April and November), with most rainfall experienced during the month of April (about 4.8 inches) and least rainfall in July (about 1.0 inches) [22]. The climate in the district is warm with temperatures ranging from 56°F to 83°F and rarely below 53°F or above 89°F, over the course of the year.
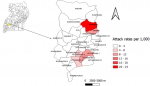
The outbreak in Kazo County in Kiruhura District during May 2018: human anthrax cases (onsets on May 17th, 2018-May 25, 2018) were reported in five villages in Kazo County (Figure 1) and these included: Kitongore I, Kitongore II, Bwera, Imiramiringa and Kakindo. Relatedly, the outbreak in livestock in Kiruhura District was reported to the Ministry of Health (MoH) on May 15th, 2018 and lasted two weeks. Public health measures including imposing quarantine of livestock in the affected areas, treatment of affected humans, vaccination in all farms at risk, disinfection and burying/burning of carcasses were instituted to control the outbreak in animals. The multidisciplinary team that responded to the outbreak included staff of the District Veterinary Department of Kiruhura, scientists from food and agricultural organization of the United Nations (FAO), One Health Initiative, Uganda Virus Research Institute (UVRI), livestock disease surveillance unit at national animal disease diagnostics and epidemiology centre (NADDEC) of the ministry of agriculture, animal industry and fisheries and Mbarara zonal agricultural research and development institute (Mbarara ZARDI) of the National agricultural research organization (NARO) and the ministry of health (MOH). Kiruhura District public health officials (district surveillance team) traced all residents that came into contact with dead anthrax-infected cattle or their meat. These included individuals that participated in butchering, handling or eating meat from the anthrax-infected cattle from May 15, 2018-May 25th, 2018, In total, 1,497 residents who got into contact with the dead cattle or meat from the anthrax-infected cattle received prophylactic antibiotics during the outbreak. There were no human deaths recorded. At least 35 cattle are estimated to have died during the outbreak at 16 farms [23].
Case definition and selection of participants: we conducted an unmatched case control study. We defined a case of human anthrax as having had anthrax infection reported to be diagnosed by a healthcare worker [7] in a resident of Kazo County from May 1st, 2018 to June 1st, 2018. We defined a control as a resident of Kazo County who was not diagnosed with anthrax infection by a healthcare worker from May 1st, 2018 to June 1st, 2018, and who had no symptoms suggestive of human anthrax (fever, ulcers, vomiting, abdominal pain, difficult in swallowing) in May 2018. Neighbors to case-persons in the nearest randomly selected households were recruited as controls. We excluded controls less than 12 years, as these could not accurately answer questions. We conducted active case finding in the community based on the constructed case definition, with the guidance of the village health team members (community health workers) and recruited all cases that could be located during the study period. In order to obtain more information on the type of treatment and case-persons´ symptoms and signs, we also reviewed patients´ records at Engari Community Health Centre (located within the same county as the study villages). Engari Community Health Center was the designated treatment center for the human anthrax cases during the outbreak. The health center registered and treated 24 cases of human anthrax during this outbreak. We administered a structured questionnaire to capture data to supplement that initially collected by the district health officials, and the records at Engari community health center the questionnaire captured data on socio-demographic characteristics (age, sex, occupation, marital status and level of education), clinical and exposure history (case-persons´ symptoms, date of onset of symptoms, exposure to and consumption of meat of carcasses of anthrax-infected animals) and knowledge about anthrax. The collection of data for this study was done from March 11th, 2019 to April 1st, 2019.
Sample size and statistical analysis: the sample size of 106 (27 cases and 79 controls) participants was calculated using Fleiss formula for unmatched case control studies in Epi Info (version 7.1.4.0, CDC, Atlanta US) on the basis of the following assumptions: statistical power of 80%, ratio of three controls to one case, 50% of controls and 80% of cases exposed, and two-sided confidence level of 95%. The exposure variable of interest in the sample size calculation was consumption of anthrax-infected meat. The information on proportions of cases and controls exposed was sourced from the data available from the local authorities during the outbreak investigation. Data were entered in EpiData 3.1 software (EpiData, Odense, Denmark), then exported to STATA version 13 (StataCorp, College Station, Texas, USA) for analysis. Data of social, demographic, behavioral characteristics and risk factors were compared by case status using Chi-square (χ2) or Fischer´s exact tests. Existence of associations was quantified with simple and multivariable logistic regression. Differences between nonparametric variables (expressed as median, range) were compared using Wilcoxon rank-sum test. Variables associated with P-value = 0.2 in the univariable analysis were entered into multivariable logistic regression models through backward stepwise elimination method to obtain the final predictive model of variables that were independently associated (P<0.05) with contracting human anthrax. We assessed the multivariable model for collinearity using variance inflation factor (VIF). This is because participants were more likely to be involved in more than one exposure task. Highly correlated variables (with VIF>5), were eliminated from the final multivariable model. The attack rate was determined as the total number of cases of human anthrax divided by the total population at risk (all the residents in households of the cases and those who came into contact with or consumed meat from dead anthrax-infected animals) expressed as a percentage.
Ethical considerations: the approval to conduct the study was obtained from and granted by Mbarara University of Science and Technology Research Ethics Committee (MUST-REC) under Reg. No.15/12-18. In the district, permission to conduct the study was granted by the District Health Officer while a written informed consent was sought from the household heads prior to interview of each household member. All adult participants (18 years and above) and parents or guardians of the minors (below 18 years) provided written informed consent. Additionally, assent was obtained from children who were below 18 years. Participants who could not write provided consent using a thumbprint. We respected the guidelines of Helsinki and CIOMS-2002 (Council for International Organizations of Medical Sciences) regarding research with humans, avoiding any type of physical or moral damage.
Social and demographic characteristics of study participants: the social and demographic characteristics of study participants are presented in Table 1. The demographic characteristics from the 101 study participants (28 cases and 73 controls), the median age of the study participants was 34 years (IQR; 26, 47). Most of the participants were female (61.4%), had ever attended school (73.3%), were subsistence farmers (85.2%) and married/living with partners (64.4%). The cases were significantly of male sex (82.1%; P<0.001), and butcher occupation (89.3%; P<0.001). The proportion of participants who were involved in slaughter of anthrax-infected animals and consuming of meat from the dead ones was significantly higher among cases compared to controls (P<0.001).
Clinical characteristics of case-persons with anthrax: the primary cases of human anthrax were recorded on May 17, 2018 as shown in the epidemic curve (Figure 2). The median duration of symptoms was 21 days (IQR; 14, 43). As shown in Table 2, the most common clinical manifestations were: having a wound/ulcer (96.4%), fever (96.4), edema (92.9%) and erythema (78.6%). A total of 22/28 (8.6%) had regional lymphadenopathy, with painful swallowing and abdominal pain recorded in 53.6% and 57.1% of the cases, respectively.
Attack rate of anthrax among residents in affected villages of Kazo County, Kiruhura District: among the 1,497 persons that came into contact with anthrax-infected meat in the five study villages, we identified 28 cases of human anthrax, for an attack rate of 1.9% among individuals who came into contact with anthrax-infected meat. There were no human fatalities reported.
Factors influencing transmission of anthrax among residents in Kazo County, Kiruhura District: the risk factors for contracting human anthrax at univariable and multivariable analyses are presented in Table 3. At univariable analysis, the categories of individuals who were significantly more likely to contract human anthrax included sex (P<0.001), occupation (P=0.004), involvement in slaughter of the animals (P<0.001), handling of meat of dead animals or their products [P<0.001)'(carrying (P<0.001) and selling hides/skins (P=0.002)) and consumption of meat from dead animals (P=0.008). At multivariable logistic regression analysis, being male was strongly associated with human anthrax (aOR=12.8; 95%CI: 3.31-49.1, P<0.001), just as slaughter of dead animals (aOR=5.74; 95%CI: 1.39-23.8, P=0.016)). Likewise, there was an association between the consumption of meat from dead animals and the disease in humans (aOR=6.4; 95%CI: 1.53- 6.7, P=0.011).
Anthrax still poses a threat to public and veterinary health in Uganda. In the current study, male sex, and exposure to anthrax-infected meat during slaughtering/butchering or consumption of the meat, were the key risk factors for contracting the disease in humans. The overall attack rate (1.9%) reported in our study varied from that in earlier literature. For instance, a study in north Zimbabwe reported an overall attack rate of 5% [24]. Comparatively, a study in a tribal village in West Bengal reported an attack rate of 7% [25], while much higher attack rates (60-67%) were reported among contacts of anthrax infected livestock in Kazakhstan [26]. In Arusha region of Tanzania an incidence of 7.9 human anthrax cases was reported per 100,000 population, while an incidence of 6.6 per 100,000 population was observed in Kilimanjaro region in the same country [2]. The lower attack rate in our study can probably be attributed to prompt effective control measures instituted to control the outbreak within livestock and human population. For instance, more than 1,000 residents that had been exposed to the dead anthrax-infected animal meat received prophylactic antibiotics. Moreover, the outbreak in livestock, which acted as a point source for the human anthrax outbreak only lasted a short period (two weeks). In the current study, slaughtering of dead anthrax-infected animals and consumption of anthrax-infected animal meat influenced the contracting of human anthrax. This is in agreement with findings of several studies that have reported exposure to infected animals or animal products to be a major risk factor for human anthrax [18,24,26,27].
Humans usually get infected from processing contaminated animal products or contact with sick animals or by insect bites [1]. Although skin abrasions were thought to be essential for cutaneous infection, the anthrax-causing organisms also may be able to invade hair follicles [28]. Additionally, the risk for human cutaneous anthrax may be exacerbated by the existence of cuts on body parts such as hands at the time of contact with the infected animal or animal products [18,26]. In the present study, most of the cases (96.4%) had clinical manifestations of the cutaneous form of human anthrax, further highlighting the effect of a high level of direct exposure to infected animals or their products. Moreover, the epidemic curve portrays a common source type of outbreak with cases rising rapidly and reaching a peak on May 20, 2018 (six days after the slaughter of anthrax-infected cattle), then dropping back to zero on May 23rd, 2018. This is within the incubation period of the disease which generally ranges from one to six days for gastrointestinal form but can reach a maximum of 12 days for cutaneous anthrax [1,29]. The fact that the animal and human samples were confirmed in the laboratory as positive for B. anthracis at Uganda Virus Research Institute (UVRI), further supports our epidemiologic linkage [23]. The existence of clinical features of gastro-intestinal form of anthrax (abdominal pain, painful swallowing and vomiting) among some of the case-persons further suggests co-occurrence of both cutaneous and gastro-intestinal forms of the disease, following contact with and consumption of contaminated meat.
The current study also found that males had significantly higher odds of contracting anthrax compared to females. Previous studies have also reported males to have at higher risk than females in contracting human cutaneous anthrax [1,25,30,31]. Gender roles may influence occupational exposure, with males being more engaged in slaughtering/butchering livestock and females more involved in cooking meat [30]. In contrast, no gender differences in the risk to anthrax were reported in studies done in Turkey and Kazakhstan [26,32]. The males were the most affected because of their involvement in slaughtering of the dead animals, rendering them more vulnerable to the disease. However, it is worth mentioning that our study captured very few female cases; therefore, the results from this study may lack the efficiency that other type of designs might have to better estimate the risk in this group. Our findings point towards the need to routinely vaccinate livestock against anthrax; notably, none of the participants had their livestock vaccinated prior to the outbreak. Thus, the control of future human anthrax outbreaks in the district will necessitate controlling the disease in livestock through improving coverage of livestock anthrax immunization; this will require embracing One Health approaches. Second, there is need to continue sensitizing the community with emphasis on safe/proper disposal of animal carcasses, avoiding consumption of meat from animals which have died from unidentified causes and decontamination of places where animals have died. Lastly, One Health approaches aimed at addressing anthrax outbreaks should consider the influence of gender on the disease vulnerability [33].
Our findings are subject to a few limitations worth mentioning. Firstly, we used report of anthrax diagnosis to define a case rather than laboratory confirmation. This may bring about misclassification of cases and controls. Nonetheless, we further cross-checked the medical forms of the case-persons, and used community health workers who were involved in the outbreak response and knew the case-persons. We also used controls who were asymptomatic during the outbreak, to further minimize chances for misclassification. Additionally, there was no major discrepancy in terms of numbers between the cases found in the community (n=28) and those recorded at Engari Community Health Center (n=24) that served as the main treatment health unit for the case-persons. Second, the study could be prone to recall bias because participants were asked about exposure to deceased animals or their products, as well as disease symptoms, that occurred more than 10 months prior to the interview date. Lastly, the study was based on relatively small numbers which impacted on some results of the multivariable analysis and some estimates had very wide confidence intervals.
The point source outbreak in humans was predominantly of the cutaneous form as a result of contact with anthrax infected meat; males had increased risk for contracting the disease. We recommend community sensitization on safe disposal of carcasses, avoiding slaughtering/handling of carcasses, and carrying out routine livestock vaccinations against anthrax in Uganda to avert similar outbreaks in future. Persons involved in slaughter of sick or dead animals from unknown causes should be educated to wear personal protective equipment during the process.
Funding: the study was financially supported by First Mile Community Health program of Mbarara University of Science and Technology (MUST) and One Health Central and Eastern Africa (OHCEA).
What is known about this topic
- Human cutaneous anthrax outbreaks in humans are associated with handling of anthrax infected animals;
- Recurrent anthrax outbreaks in humans are associated with low vaccination coverage against anthrax in livestock.
What this study adds
- It highlights co-existence of both cutaneous and gastro-intestinal forms of human anthrax following contact with and consumption of anthrax infected meat;
- It acknowledges the influence of gender differences on vulnerability to zoonotic infections such as anthrax;
- It emphasizes the need for a One Health approach to avert similar anthrax outbreaks among humans in future.
The authors declare no competing interests.
Richard Migisha, David Collins Agaba, Aggrey Byaruhanga, Gabriel Tumwine, and Halid Kirunda, contributed to the design and implementation of the research; Richard Migisha and Eleanor Turyakira analyzed the data; Gad Ndaruhutse Ruzaaza, Gabriel Tumwine, Aggrey Siya, Aggrey Byaruhanga, and Halid Kirunda. provided support interpreting the findings and writing the manuscript. All the authors have read and agreed to the final manuscript.
We thank the study participants and the staff of Engari Community Health Center who contributed in various ways towards the success of the study. We are very grateful to the district health team of Kiruhura District Local Government for providing us the necessary support for the success of the study. We also acknowledge the contribution of Dr. Grace Asiimwe and Dr. Ivan Kamya of Kiruhura District Local Government for their support in conducting the study.
Table 1: baseline socio-demographic characteristics, and exposures of study participants by case
Table 2: clinical characteristics of case-persons with anthrax in Kazo County, Kiruhura District
Table 3: risk factors associated with contracting human anthrax
Figure 1: attack rates by villages per 1,000 persons in Kazo County, Kiruhura District, southwestern Uganda, May 2018 (map drawn using QGIS browser 3.10.2)
Figure 2: distribution of symptom onset date of 28 case-persons during the outbreak: Kazo County, Kiruhura District, Uganda, May 2018
- World Health Organization. Anthrax in humans and animals. World Health Organization. 2008. Google Scholar
- Mwakapeje ER, Sol H, Robert F, Hezron EN, Robinson HM, Eystein S et al. Anthrax outbreaks in the humans-livestock and wildlife interface areas of Northern Tanzania: a retrospective record review 2006-2016. BMC Public Health. 2018 Jan 5;18(1):106 PubMed | Google Scholar
- Mwakapeje ER, Sol H, Adis S, Janneth M, Hezron EN, Robinson HM et al. Risk factors for human cutaneous anthrax outbreaks in the hotspot districts of Northern Tanzania: an unmatched case-control study. R Soc Open Sci. 2018 Sep 5;5(9):180479 PubMed | Google Scholar
- Hang'ombe MB, James CLM, Sergio M, Phillip M, Muzala K Eric M et al. Human-animal anthrax outbreak in the Luangwa valley of Zambia in 2011. Trop Doct. 2012 Jul;42(3):136-9. PubMed | Google Scholar
- National Research Council (US) Committee on achieving sustainable global capacity for surveillance and response to emerging diseases of zoonotic origin. Achieving sustainable global capacity for surveillance and response to emerging diseases of zoonotic origin: workshop summary. National Academies Press. 2008. PubMed | Google Scholar
- Molyneux D, Zuhair H, Gerald TK, Donald PM, Helena N, Sarah C et al. Zoonoses and marginalised infectious diseases of poverty: where do we stand? Parasites and vectors. 2011;4(1):106. Google Scholar
- Lehman, MW, Allen SC, Constantine M, Kapina-Kany'anga M, Philip M, Fanny M et al. Role of food insecurity in outbreak of anthrax infections among humans and hippopotamuses living in a game reserve area, rural Zambia. Emerg Infect Dis. 2017 Sep;23(9):1471-1477. PubMed | Google Scholar
- Blackburn JK, Kristina MM, Andrew C, E Hugh-Jones Martin. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am J Trop Med Hyg. 2007 Dec;77(6):1103-10. PubMed | Google Scholar
- Kracalik I, Abdullayev R, Asadov K, Ismayilova R, Baghirova M, Ustun N et al. Changing patterns of human anthrax in Azerbaijan during the post-Soviet and preemptive livestock vaccination eras. PLoS neglected tropical diseases. 2014;8(7):e2985. Google Scholar
- Carlson CJ, Ian TKk, Noam R, Kathleen AA, E Hugh-Jones Martin, Mark F et al. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat Microbiol. 2019 Aug;4(8):1337. PubMed | Google Scholar
- Munyua P, Austine B, Eric O, Emily GP, Josephat M, Athman M et al . Prioritization of zoonotic diseases in Kenya, 2015. PLoS One. 2016 Aug 24;11(8):e0161576. PubMed | Google Scholar
- Sekamatte M, Krishnasamy V, Bulage L, Kihembo C, Nantima N, Monje F et al. Multisectoral prioritization of zoonotic diseases in Uganda, 2017: a one health perspective. PloS one. 2018;13(5):e0196799. Google Scholar
- Jones KE, Nikkita GP, Marc AL, Adam S, Deborah B, John LG et al. Global trends in emerging infectious diseases. Nature. 2008 Feb 21;451(7181):990-3 PubMed | Google Scholar
- Uganda Bureau of Statistics. The national population and housing census 2014- main report. Kampala.
- Opare C, Nsiire A, Awumbilla B, Akanmori BD. Human behavioural factors implicated in outbreaks of human anthrax in the Tamale municipality of northern Ghana. Acta Trop. 2000 Jul 21;76(1):49-52. PubMed | Google Scholar
- Blas E, Kurup AS. Equity, social determinants and public health programmes. World Health Organization. 2010. Google Scholar
- Siamudaala VM, John MB, Hetron MM, Peter GS, Fred B, Aaron SM et al. Ecology and epidemiology of anthrax in cattle and humans in Zambia. Japanese Journal of Veterinary Research. 2006;54(1):15-23. PubMed | Google Scholar
- Gombe NT, Nkomo BMM, Chadambuka A, Shambira G, Tshimanga M et al. Risk factors for contracting anthrax in Kuwirirana ward, Gokwe North, Zimbabwe. African Health Sciences. 2010;10(2):159-164. Google Scholar
- Kiruhura district Local government, Kiruhura District Local Government statistical abstract. Kiruhura, Uganda. 2012.
- Uganda Bureau of Statistics. National population and housing census 2014 area specific profiles-Kiruhura District. Kampala, Uganda. 2017.
- Kiruhura District Local Government. Administrative units of Kiruhura District Local Govt. 2019 3rd July.
- Weather Spark. Average weather in Kiruhura 2020. Accessed March 3, 2020.
- World Health Organization. Weekly bulletin on outbreaks and other emergencies; health emergency information and risk assessment. WHO. 2018. Google Scholar
- Chirundu D, Chihanga S, Chimusoro A,Chirenda J, Apollo T,Tshimanga M. Behavioural factors associated with cutaneous anthrax in Musadzi area of Gokwe North, Zimbabwe. Cent Afr J Med. Sep-Dec 2009;55(9-12):50-4. PubMed | Google Scholar
- Chakraborty PP, Sudeshna GT, Partha SS, Shukchand H, Sudipta S, Arun A et al. Outbreak of cutaneous anthrax in a tribal village: a clinico-epidemiological study. J Assoc Physicians India. 2012 Feb;60:89-93. PubMed | Google Scholar
- Woods CW, Kenes O, Akylbek M, Michael F, Brian P, David AA. Risk factors for human anthrax among contacts of anthrax-infected livestock in Kazakhstan. Am J Trop Med Hyg. 2004 Jul;71(1):48-52. PubMed | Google Scholar
- Kasradze A, Diana E, Khatuna Z, Christian B, Nicholas H, Paata I et al. Rates and risk factors for human cutaneous anthrax in the country of Georgia: national surveillance data, 2008-2015. PLoS One. 2018 Feb 7;13(2):e0192031. PubMed | Google Scholar
- Zakowska D, Micha B, Marcin N, Bielawska-Drózd A, Janusz K. New aspects of the infection mechanisms of B anthracis. Ann Agric Environ Med. 2012;19(4):613-8. PubMed | Google Scholar
- Heymann DL. Control of communicable diseases manual. American public health association. 2009 15 Octo;49(8):1292-1293.
- Kracalik I, Lile M, Nikoloz T, Julietta M, Lela B, Paata I et al. Human cutaneous anthrax, Georgia 2010-2012. Emerg Infect Dis. 2014 Feb;20(2):261-4. PubMed | Google Scholar
- Gurbanov S, Akhmedova S. Especially dangerous infections in Azerbaijan, in emerging and endemic pathogens. Springer. 2010;39-43. Google Scholar
- Doganay M, Metan G. Human anthrax in Turkey from 1990 to 2007. Vector Borne Zoonotic Dis. 2009 Apr;9(2):131-40. PubMed | Google Scholar
- FAO, O, WHO. A tripartite guide to addressing zoonotic diseases in countries. World Health Organization (WHO), Food and Agriculture Organization of the United Nations (FAO) and World Organisation for Animal Health (OIE). 2019.