Prevalence and sensitivity to antibiotics of Campylobacter spp. in chicken, farmers and soil in Bobo-Dioulasso, Burkina Faso
Abdul Gafar Victoir Coulidiaty, Adama Sanou, Clarisse Abikpo Houngbedji, Diakourga Arthur Djibougou, Amadou Dicko, Gnada Kobo, Banavona Mélaine Kambou, Eli Kabré, Abdoul-Salam Ouedraogo, Bassirou Bonfoh
Corresponding author: Abdul Gafar Victoir Coulidiaty, Centre Muraz, Bobo-Dioulasso, Burkina Faso 
Received: 27 Jan 2021 - Accepted: 08 Feb 2021 - Published: 09 Feb 2021
Domain: Bacteriology,Food safety,Zoology
Keywords: Campylobacter, chicken, farming, antibiotic resistance, Burkina Faso
©Abdul Gafar Victoir Coulidiaty et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Abdul Gafar Victoir Coulidiaty et al. Prevalence and sensitivity to antibiotics of Campylobacter spp. in chicken, farmers and soil in Bobo-Dioulasso, Burkina Faso. PAMJ-One Health. 2021;4:8. [doi: 10.11604/pamj-oh.2021.4.8.28089]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/4/8/full
Research 
Prevalence and sensitivity to antibiotics of Campylobacter spp. in chicken, farmers and soil in Bobo-Dioulasso, Burkina Faso
Prevalence and sensitivity to antibiotics of Campylobacter spp. in chicken, farmers and soil in Bobo-Dioulasso, Burkina Faso
![]() Abdul Gafar Victoir Coulidiaty1,2,&, Adama Sanou1,3, Clarisse Abikpo Houngbedji4,5, Diakourga Arthur Djibougou1,
Abdul Gafar Victoir Coulidiaty1,2,&, Adama Sanou1,3, Clarisse Abikpo Houngbedji4,5, Diakourga Arthur Djibougou1, ![]() Amadou Dicko1,
Amadou Dicko1, ![]() Gnada Kobo1, Banavona Mélaine Kambou1,2, Eli Kabré1, Abdoul-Salam Ouedraogo3,6, Bassirou Bonfoh5
Gnada Kobo1, Banavona Mélaine Kambou1,2, Eli Kabré1, Abdoul-Salam Ouedraogo3,6, Bassirou Bonfoh5
&Corresponding author
Introduction: campylobacteriosis is zoonotic and one of the leading causes of bacterial foodborne diarrheal disease worldwide. Data on Campylobacter is scarce in Burkina Faso and do not provide information on the growing anti-microbial resistance (AMR) in the poultry value-chain. This study aims to assess the prevalence of Campylobacter spp. simultaneously in poultry, in farmers and soil, as well as its impact on the emergence of AMR.
Methods: qualitative survey data provided information on the antibiotics used as breeding promoters in poultry farming. Campylobacter spp. strains were obtained from cloacal swabs, farmers´ stools and soil samples. Isolation, identification, and drug sensitivity tests were performed in the bacteriology laboratory of the Centre Muraz using the automated Vitek 2 ID/AST, Biomerieux and Kirby Bauer method.
Results: the antibiotics used by farmers included: Oxytetracycline, Sulfadimidine, Colistine, Trimazin, Erythromycin, Streptomycin and Colistin. The prevalence of campylobacter in poultry cloacal swabs was 4.44% (95% CI 1.4%, 7.5%). No campylobacter was isolated from human stools and soil samples. Three different species were isolated: Campylobacter jejuni (62.5%), Campylobacter fetus (25%) and Campylobacter coli (12.5%). From these species 37.5% were found to be resistant to ciprofloxacin and nalidixic acid, 50% to ampicillin and 87.5% to tetracycline.
Conclusion: while the resistance of campylobacter to antibiotics relevant to public health was considerable, the overall antibiotic resistance was lower than expected considering the generous use of antimicrobials in poultry breeding.
Campylobacter spp. is recognized as the leading cause of bacterial foodborne diarrheal disease worldwide [1-3]. Since 2005, human campylobacteriosis has been the most frequently reported foodborne bacterial gastrointestinal disease in Europe [4,5]. Food animals, particularly poultry, are the main reservoirs of campylobacter which also serve as the main sources for human infection. The transmission of campylobacteriosis is through the ingestion of contaminated meat, animal products or water. Poultry dejections (feces) are therefore a potential source of pathogens for farmers and the soil [5,6]. Indeed around 50-80% of sporadic human campylobacter infections are attributable to contact with, or handling of, live poultry and consumption of poultry, especially undercooked. A recent case-control study identified chicken consumption as source of infection for 24-29% of the 303 cases [5]. There is also a concern regarding the development of antibiotic resistance of campylobacter due to the use of antimicrobials in intensive animal breeding especially in poultry farming. Among the resistant microorganisms that can transmit anti-microbial resistance (AMR), campylobacter species are of high concern.
They are among the nine (9) bacteria for which AMR is of particular public health concern according to the World Health Organization (WHO) [7,8]. Campylobacter from poultry has also been shown to be of particular relevance in AMR development in humans; for example, various studies have reported an increase in Campylobacter resistance to antimicrobials, particularly to fluoroquinolones [6,9-11]. This has been observed in countries such as Italy and Japan, where all broiler strains and 92% of Turkey strains were multidrug resistant [9] and 63% of Campylobacter isolates from poultry were resistant to all three fluroquinolones authorized in poultry breeding [10]. This resistance to fluoriquinolones has also been observed in several countries in Africa, such as Nigeria, where resistance was reported to be between 39% and 81% [12]. In Côte d´Ivoire, Gblossi et al. [13] found antimicrobial resistance of Campylobacter isolates to nalidixic acid and ciprofloxacin of 78.9% and 50%, respectively. Resistance was also observed for other classes of antibiotics. Outside Africa, in Australia, a study on Campylobacter in poultry found 51-100% resistance of the bacteria to Lincomycin, extensive resistance to Ampicillin (33-60%) and some resistance to tetracycline (5.6-40%) [11]. In Côte d´Ivoire, Gblossi et al. [13] found the prevalence of resistance to amoxicillin, erythromycin and gentamicin at 17.9%, 10.3%, and 7.7% for C. jejuni and 13.5%, 8.1% and 0% for C. coli respectively.
Massive use of antibiotics as growth promoters and for infection prevention is a serious risk factor for the development of antibiotic resistance and its spread in livestock breeding systems. The use of antibiotics can exacerbate the occurrence of drug resistance and resistant bacteria can be transmitted to humans through poultry product handling and consumption. This in turn can give rise to a novel strain of resistant bacteria in humans. This may lead to morbidity and mortality and thus is a serious threat for public health [8,14].
While the prevalence and risk factors of Campylobacter infections have been well documented in developed countries [5,11,15,16], Africa and particularly Burkina Faso, lack comprehensive and representative data. This is despite the livestock and agricultural sector representing around 80% of the socio-economic activity. Poultry farming is a major contributor to food security and specifically responds to the protein needs of the urban population. A large diversity of antimicrobials is used to raise poultry in Burkina Faso. Despite availability of data on prevalence of Campylobacter in poultry in Ouagadougou, Burkina Faso [17] we do not have data on antibiotic uses in poultry breeding nor the consequences on antibiotic resistance and spread. Campylobacter data linking human, animal and environment is not established. This study aimed to assess the use of antibiotics in poultry breeding, isolate Campylobacter strains and test their antibiotic sensitivity at the human-poultry-soil interface. The data provide insight on the risk factors and avenues for safeguarding public health.
Study area and design: a cross-sectional study took place in urban and peri-urban areas of Bobo-Dioulasso city from May to July 2019. Bobo-Dioulasso is an economic, industrial and cultural center in Burkina Faso and located at 365 km southwest from the capital Ouagadougou.
Survey and sample collection: a total of 22 farms were selected randomly and included in the study (11 modern farms and 11 traditional ones in Bobo-Dioulasso). Three farms of each type (modern and traditional) from each of the 3 main parts and 2 in the city of Bobo-Dioulasso were selected in a random manner after the list of farms of these areas was obtained. Modern farms were characterized by poultry mass-housing facilities and permanent employees while a traditional farm was characterized as providing basic free-range conditions and simple housing for their birds.
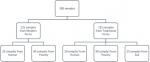
The study budget allowed for the collection and processing of a total of 260 samples. One hundred eighty (180) of these were cloacal swabs from poultry, with 90 from modern farms and 90 from traditional farms; 55 were stool samples from humans (20 from traditional farms and 35 from modern farms); and finally, 25 were soil samples from the households where poultry were raised in the traditional way and therefore came in contact with soil as shown in Figure 1. All the poultry from traditional breeding were local breeds and all those from modern farms were non-local breeds.
Poultry farmers and their staff were interviewed with a questionnaire translated into the local language. The antibiotics used in poultry were classified according to the active compound in the product. A list of antibiotics authorized by the livestock sector was obtained from the ministry of animal and fisheries. We collected samples from the modern (layers and broilers) and traditional poultry farms. The traditional farms were chosen near (1 to 2 km) the modern ones and soil samples were collected from the poultry breeding space in the traditional farms.
As for poultry sample collection, cloacal swabs were taken and immediately stored in tubes containing distilled water (sterile physiological water). The human stool samples (first bowel movement of the day) were collected from each consenting farmer or farmyard keeper in a sterile, clean and dry container with a screw cap. The environmental samples consisted of 2 g top soil in a sterile tube. All the collected samples were transported, within 2 hours of sampling in a cool box to the bacteriology laboratory of Centre Muraz for immediate analyses.
Isolation and identification of Campylobacter strains: animal and human samples were inoculated on the modified charcoal-cefoperazone-deoxycholate agar (mCCDA) supplemented with selective supplement (SR0155; Oxoid). Soil samples were dissolved in sterile physiological water and a small volume was used for culture as describe above. All samples (animal, human and environmental) were grown on a media incubated at 37°C under micro aerobic conditions generated by a gas-generating pack (Campygen CN25; Oxoid) for 48 hours [9,10,13,17]. Each reagent was tested on the reference sample donated by the Centre de Recherche Clinique de Nanoro, Burkina Faso, prior to its use on the study samples.
Suspicious growth was morphological identified as small, smooth, radiant, flat or convex colonies, confluence on fresh media. The biochemical characteristics and the aspects of the bacterium under microscopical examination were recorded after gram staining. Finally, isolates with similar characteristics to Campylobacter were identified using the compact Vitek 2 automat in accordance with the manufacturer's instructions [18,19].
Antibiotic susceptibility test of Campylobacter isolates: the identified strains were sub-cultured onto Columbia agar medium with 5% sheep blood and incubated at 42°C for 24h. Antibiotic susceptibility testing (AST) was performed using the Kirby Bauer method on Mueller Hinton agar (MHA) supplemented with 5% sheep blood. Briefly, three to five well-isolated colonies were selected and transferred into Trypticase Soy Broth (TSB). After incubation at 42°C for 24 hours under microaerobic conditions, the suspension turbidity was adjusted to 1.0 McFarland. The bacterial suspension was then swabbed uniformly across a culture plate under aseptic conditions. Seven antibiotic discs were used: tetracycline (30 µg), erythromycin (15 µg), gentamicin (10 µg), nalidixic acid (30 µg), ciprofloxacin (5 µg), Ampicillin (10 µg) and amoxicillin-clavulanic acid (20 µg/10 µg) (neo sensitabstm, rosco-diagnostica, Denmark). The discs were carefully placed with sterile tweezers onto the seeded bacterial lawn on the surface of the medium and the plates were incubated under a microaerophilic atmosphere at 42°C for 24 to 48 hours. Zones of growth inhibition were measured and the isolate susceptibility to a given antibiotic was evaluated according to the manufacturer´s instructions and European Committee on Antimicrobial Susceptibility Testing (EUCAST) [20].
Statistical analysis: prevalence was calculated using a calculator. Chi-square tests were used to evaluate association between the poultry breeding system (modern, traditional) and the campylobacter species with 95% level of confidence.
Ethical considerations: ethical approval was obtained from the ethics committee of Centre Muraz research institute prior the starting of the study, permit number 2019-17 of 26th March 2019. The aims of the study were explained to the farm owners and their staff and informed written consent was obtained before they were interviewed in the local language. All information given was anonymized.
Use of antibiotics in poultry farms: type, dosage and frequency of administration of antibiotics used was not documented by farmers (no record, no empty packaging), mostly due to illiteracy. An unspecified amount of antibiotic was ground to a powder by farmers and added at an unspecified and unmeasured quantity to poultry drinking water for a variable unknown number of days. It was therefore impossible to estimate dosage absorbed per animal. However, all the breeders, in modern and traditional farms admitted their usage (we showed them samples and pictures of the drugs), especially oxytetracycline. The antibiotics reported included: oxytetracycline, sulfadimidine, colistine, trimazin, erythromycin, streptomycin; colistin all breeders were buying their antibiotics from small drug shops, mostly from informal sector.
Antibiotics authorized in Burkina Faso: there are 4 families of antibiotics authorized by the veterinary authorities in Burkina Faso in poultry breeding. They are: Tetracyclines, Fluoroquinolones, Sulphonamides (Cotrimoxazole) And Polypeptides (colistin). None of the participants was aware of the existence of such a list.
Prevalence of Campylobacter: out of the 260 samples examined, we identified 8 isolates of Campylobacter giving an overall prevalence of 3.4%, 95% CI [1%, 5.2%]. No strain was isolated from human stools and soil samples. The prevalence in poultry samples alone was 4.44% 95% CI [1.4%, 7.5%]. The 8 Campylobacter strains identified belong to three different species: Campylobacter coli (1 isolate), Campylobacter fetus (2 strains) and Campylobacter jejuni (5 isolates). Among these isolates seven were from modern farms and one from a traditional farm (Table 1). Modern farms were positively associated with the occurrence of Campylobacter (χ2=8.372, p-value=0.00381046).
Campylobacter resistance to antibiotics: all 8 isolates were sensitive to amoxicillin-clavulanic acid, gentamycin and erythromycin. The resistance rates were 37.5% (for ciprofloxacin and nalidixic acid), 50% for ampicillin and 87.5% for tetracycline (Table 2). Only the sample from traditional breeding was sensitive to tetracycline.
This study was a pilot searching for Campylobacter in this part of Burkina Faso, the results give insights on the prevalence of Campylobacter and its sensibility to antibiotics and practices of farmers in terms of usage of antibiotics. The main limitation is lack of usage of molecular biology technics. However, for the first time, C. jejuni, C. fetus and C. coli species were isolated from poultry in Bobo-Dioulasso and showed high variability in resistance to antibiotics. No strains were isolated from human stool or farm soil samples.
Only strains with catalase and oxidase were targeted as they are the most often observed in Campylobacter species encounter in human pathology. The estimated prevalence (4.4%) of Campylobacter was low compared to other studies. For the moment, there is no study that looked at prevalence of Campylobacter based on poultry cloacal swab sampling in Burkina Faso; whose results could be compared to our results. In other African countries, the prevalence was found to be high, between 33 and 44% in Kenya, Nigeria and Cote d´Ivoire [12,13,21,22]. These prevalence were even higher in Europe and the Middle East; where it reached 64% in Poland [23], 40% in Belgium [16] and 64% in Iran [24]. In other African countries, the prevalence was also found to be high, between 33 to 44% in Kenya, Nigeria and Cote d´Ivoire [12,13,21,22]. The low prevalence of Campylobacter in our study could be explained by the low rate of intensification/modernization of poultry farming in Burkina Faso where traditional farming is the main provider of poultry meat. It seems to have an association between the type of poultry farm and the isolation of Campylobacter was established as the bacterium was mainly isolated from chicken raised in modern farms (χ2=8.372, p-value=0.0038), which have other breeds and a much higher density. In addition, Kagembega et al. found a higher prevalence (67.96%) in Ouagadougou, 17 times higher than what we found. However, the authors collected the samples from the chicken carcasses-selling stalls where the carcasses may have been contaminated due to lack of hygiene.
The AST highlighted the presence of antibiotic-resistant isolates. One of the possible causes of the development of antibiotic resistance is the use of most of these molecules as growth promoters in poultry farming [1]. That is why a comprehensive survey on the use of antibiotics in poultry farming in this area would add essential information. Several molecules, such as macrolides and fluoroquinolones [3] are globally used for this purpose with practices that can increase the resistance of bacteria hosted by poultry especially Campylobacter isolates which are an important pathogen affecting public health. The use of several molecules as promoters in poultry farming in Bobo-Dioulasso is regulated but policing the adherence to the regulation is seldom performed. An official list of antibiotics allowed to be used in poultry breeding in Burkina Faso is available, but none of the breeders were aware of this. There are 4 families of antibiotics authorized for use in poultry, tetracyclines, fluoroquinolones, sulphonamides (cotrimoxazole) and polypeptides (colistin). The antibiotics used by poultry breeders were not clearly documented on any of the farms and there was no register where the antibiotic administration was followed up.
In this context, our study found a resistance rate of 37.5% to fluoroquinolones in both of the tested molecules (ciprofloxacin and nalidixic acid) in the same isolates. This prevalence is lower than what is encountered in the rest of the world. Ciprofloxacin resistance has been reported in developing countries with levels ranging from 30 to greater than 84% [3]. The use of fluoroquinolones in poultry breeding has been clearly established to increase the resistance of Campylobacter to fluoroquinolones [1]. The low modernization of poultry breeding with less use of fluoroquinolones could explain the results obtained in our study. However, attention must be put into stakeholder´s compliance to reduce the use of fluoroquinolones as its resistance can emerge very quickly. Macrolides, such as erythromycin, inhibit protein synthesis in Campylobacter by binding to their ribosomes, causing dissociation of the peptidyl-tRNA and thereby preventing bacterial growth. Advantages of using erythromycin include the low frequency of natural resistance of Campylobacter to erythromycin [25,26].
The prevalence of Campylobacter resistance to erythromycin is very low. Out of 1,808 isolates from Finnish patients between 2003 and 2005, a resistance prevalence to erythromycin was reported for 1.1% [27]. Generally, the prevalence of erythromycin resistance among Campylobacter strains (including both C. jejuni and C. coli) isolated from humans, broilers and cattle in the USA and Canada has been reported at 10% or lower [28,29]. Likewise, macrolides resistance among Campylobacter isolates from humans and C. jejuni isolates from chickens and cattle has been low and stable in most European countries [26,28,30,31]. In our study all the strains of Campylobacter were sensitive to erythromycin. The resistance to tetracycline in this study was high (87.5%) representing double of what was found in Europe [27]. In Brazil, Campylobacter isolated from broiler samples at slaughterhouses were resistant to tetracycline at 35.5% [2] and 19.5% in the USA [32]. The high resistance to tetracycline was explained by the overuse of this molecule in the breeding process. All the modern farms included in this study are using tetracycline at an unprecise dosage. The pressure has probably selected resistant strains in all the sample from modern farms.
The transmission of campylobacteriosis is through the ingestion of contaminated meat, animal products or water. Poultry dejections are therefore a potential source of contamination for farmers who work without protective clothing when collecting eggs and catching chickens. In our study only 1 of the identified Campylobacter strains was isolated from traditionally bread poultry, representing only 1.7% of the samples obtained from these poultry. Unsurprisingly, there was also a lack of Campylobacter strain isolation from the soil where the poultry walks freely in traditional breeding systems. A probable reason for this is the low density of poultry in these systems, compared to modern poultry farms even though Campylobacter survives in soil for around 10 days, sometimes more than 32 days [33]. Surprisingly, the study failed to identify Campylobacter in the farm staff, even in the farms where Campylobacter was isolated. This result should be interpreted carefully because the samples were obtained not in a context of gastroenteritis or it might be due to good hygiene practice of the keepers. Preventive approaches such as good hygiene practices and biosecurity now find some interest and may be a strategy to prevent the colonization of animals by Campylobacter, reduce the development of antibiotic resistance and participate in the control of this zoonotic agent in the production of poultry meat. This interest is reinforced by the development of indirect measures, complementary of best practices, to reduce the intestinal load of Campylobacter in poultry [34]. However, in Burkina Faso the application of these measures is yet to be improved. In addition, the preventative measures are still very general and not pathogen specific (ministry of animal health).
This study found a low prevalence of Campylobacter from intensive poultry farming and failed to isolate Campylobacter from humans and soil samples. The resistance of the isolated strains to the clinically relevant antibiotics namely macrolides and fluoroquinolones is low, however, efforts should be made to regulate the use in order to reduce the risk of drug resistance spreading.
Despite the issue of campylobacteriosis being neglected in livestock production, food safety and public health research, it is clear that Campylobacter spp. infections and campylobacteriosis in the general population and in animal hosts as well as contamination in the environment are frequent. There is lack of epidemiological data on predominant Campylobacter spp. and systematic analysis of human and livestock risk factors which could lead to better understand the most acceptable and cost-effective intervention options that cannot be reached with fragmented studies in different sub-sectors and countries.
What is known about this topic
- Campylobacteriosis is a zoonotic disease and one of the leading causes of bacterial foodborne diarrheal disease worldwide;
- The inappropriate use of antibiotics in animals, especially in poultry has led to a threat of AMR in animal and human health.
What this study adds
- The prevalence of Campylobacter in poultry breeding sector including in poultry, environment and humans is low;
- The resistance to antibiotics of isolated strains of Campylobacter is low in general except for tetracycline where it is very high;
- The antibiotics are used inappropriately in poultry breeding in Bobo-Dioulasso.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
This study was funded by African Science Partnership for Intervention Research Excellence (Afrique One-ASPIRE). The corresponding author would like to thank the consortium for this support. The researchers also thank Centre Muraz for hosting the study and the veterinary authorities of Burkina Faso for their collaboration.
Table 1: Campylobacter species isolated from traditional and modern poultry farms
Table 2: isolated Campylobacter strains and antibiotic susceptibility tests results
Figure 1: sampling design to assess antibiotic use and Campylobacter prevalence in poultry breeding systems and their resistance profile
- Humphrey TJ, Jørgensen F, Frost JA, Wadda H, Domingue G, Elviss NC et al. Prevalence and subtypes of ciprofloxacin-resistant Campylobacter spp in commercial poultry flocks before, during and after treatment with fluoroquinolones. Antimicrob Agents Chemother. 2005;49(2):690-698. PubMed | Google Scholar
- Sierra-Arguello YM, Morgan RB, Perdoncini G, Lima LM, Gomes MJP, Nascimento VP do et al. Resistance to β-lactam and tetracycline in Campylobacter spp isolated from broiler slaughterhouses in southern Brazil. Pesquisa Veterinária Brasileira. 2015;35(7):637-642. Google Scholar
- Yang Y, Feye KM, Shi Z, Pavlidis HO, Kogut M, J Ashworth A et al. A historical review on antibiotic resistance of foodborne Campylobacter. Front Microbiol. 2019;10:1509. PubMed | Google Scholar
- Iovine NM, Blaser MJ. Antibiotics in animal feed and spread of resistant campylobacter from poultry to humans. Emerg Infect Dis. 2004;10(6):1158-1189. PubMed | Google Scholar
- Bless PJ, Schmutz C, Suter K, Jost M, Hattendorf J, Mäusezahl-Feuz M et al. A tradition and an epidemic: determinants of the campylobacteriosis winter peak in Switzerland. Eur J Epidemiol. 2014;29(7):527-537. PubMed | Google Scholar
- Iovine NM. Resistance mechanisms in Campylobacter jejuni. Virulence. 2013;4(3):230-240. PubMed | Google Scholar
- World Health Organization. Antimicrobial resistance: global report on surveillance: 2014 summary. 2014. Google Scholar
- Batonon-Alavo DI, Bastianelli D, Chrysostome Christophe AAM, Duteurtre G, Lescoat P. Sécurisation des flux d'approvisionnement en matières premières et de mise en marché des produits dans le secteur avicole: cas de la filière œufs au Bénin. Rev Elev Med Vet Pays Trop. 2015;68(1):3-18. Google Scholar
- Giacomelli M, Salata C, Martini M, Montesissa C, Piccirillo A. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli from poultry in Italy. Microb Drug Resist. 2014;20(2):181-188. PubMed | Google Scholar
- Kojima C, Kishimoto M, Ezaki T. Distribution of antimicrobial resistance in campylobacter strains isolated from poultry at a slaughterhouse and supermarkets in Japan. Biocontrol Sci. 2015;20(3):179-184. PubMed | Google Scholar
- Obeng AS, Rickard H, Sexton M, Pang Y, Peng H, Barton M. Antimicrobial susceptibilities and resistance genes in campylobacter strains isolated from poultry and pigs in Australia. J Appl Microbiol. 2012;113(2):294-307. PubMed | Google Scholar
- Nwankwo IO, Faleke OO, Salihu MD, Musa U, Magaji AA, Audu B et al. Prevalence and molecular identification of campylobacter species isolates from poultry and humans in Sokoto, Nigeria. Sokoto Journal of Veterinary Sciences. 2017;15(4):1-8-8. Google Scholar
- Gblossi Bernadette G, Eric Essoh A, Elise Solange K-N, Natalie G, Souleymane B, Lamine Sébastien N et al. Prevalence and antimicrobial resistance of thermophilic campylobacter isolated from chicken in Côte d´Ivoire. Int J Microbiol. 2012;2012:150612. PubMed | Google Scholar
- Ouedraogo B, Bale B, Zoundi SJ, Sawadogo L. Caractéristiques de l'aviculture villageoise et influence des techniques d'amélioration sur ses performances zootechniques dans la province du Sourou, région Nord-Ouest Burkinabè. International Journal of Biological and Chemical Sciences. 2015;9(3):1528-1543. Google Scholar
- Ghasemzadeh A, Jaafar HZE, Juraimi AS, Tayebi-Meigooni A. Comparative evaluation of different extraction techniques and solvents for the assay of phytochemicals and antioxidant activity of Hashemi Rice Bran. Molecules. 2015;20(6):10822-10838. PubMed | Google Scholar
- Vandeplas S, Dubois-Dauphin R, Palm R, Beckers Y, Thonart P, Thewis A. Prevalence and sources of Campylobacter spp contamination in free-range broiler production in the southern part of Belgium. Biotechnologie, Agronomie, Société et Environnement. 2010;14. Google Scholar
- Kagambèga A, Thibodeau A, Trinetta V, Soro DK, Sama FN, Bako É et al. Salmonella spp and Campylobacter spp in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Sci Nutr. 2018;6(6):1601-1606. PubMed | Google Scholar
- Biomerieux. VITEK® 2 NH ID card: identification of fastidious microorganisms. Accessed 27th March 2020.
- Rennie RP, Brosnikoff C, Turnbull L, Reller LB, Mirrett S, Janda W et al. Multicenter evaluation of the Vitek 2 anaerobe and corynebacterium identification card. J Clin Microbiol. 2008;46(8):2646-2651. PubMed | Google Scholar
- Kahlmeter G, Brown DFJ, Goldstein FW, MacGowan AP, Mouton JW, Odenholt I et al. European committee on antimicrobial susceptibility testing (EUCAST) technical notes on antimicrobial susceptibility testing. Clin Microbiol Infect. 2006;12(6):501-503. PubMed | Google Scholar
- Carron M, Chang YM, Momanyi K, Akoko J, Kiiru J, Bettridge J et al. Campylobacter, a zoonotic pathogen of global importance: prevalence and risk factors in the fast-evolving chicken meat system of Nairobi, Kenya. PLoS Negl Trop Dis. 2018;12(8):e0006658. PubMed | Google Scholar
- Salihu MD, Junaidu AU, Oboegbulem SI, Egwu GO, Magaji AA, Abubakar MB et al. Prevalence of Campylobacter spp in Nigerian indigenous chicken in Sokoto State Northwestern Nigeria. The Internet Journal of Veterinary Medicine. 2009;7(1). Google Scholar
- Anna S, Bartodziejska B, Królasik J, Paziak-Domańska B, Korsak D, Chmiela M. The prevalence of Campylobacter spp in Polish poultry meat. Pol J Microbiol. 2018;67(1):117-120. PubMed | Google Scholar
- Rahimi E, Reza Kazemeini H, Safaei S, Allahbakhshi K, Momeni M, Riahi M. Detection and identification of Campylobacter spp from retail raw chicken, turkey, sheep and goat meat in Ahvaz, Iran. African Journal of Microbiology Research. 2010;4:1620-1623. Google Scholar
- Smith KE, Besser JM, Hedberg CW, Leano FT, Bender JB, Wicklund JH et al. Quinolone-resistant campylobacter jejuni infections in Minnesota, 1992-1998: investigation Team. N Engl J Med. 1999;340(20):1525-1532. PubMed | Google Scholar
- Bardon J, Kolar M, Cekanova L, Hejnar P, Koukalova D. Prevalence of Campylobacter jejuni and its resistance to antibiotics in poultry in the Czech Republic. Zoonoses Public Health. 2009;56(3):111-116. PubMed | Google Scholar
- Lehtopolku M, Nakari U-M, Kotilainen P, Huovinen P, Siitonen A, Hakanen AJ. Antimicrobial susceptibilities of multidrug-resistant campylobacter jejuni and C. coli strains: in vitro activities of 20 antimicrobial agents. Antimicrob Agents Chemother. 2010;54(3):1232-1236. PubMed | Google Scholar
- Gibreel A, Taylor DE. Macrolide resistance in Campylobacter jejuni and Campylobacter coli. J Antimicrob Chemother. 2006;58(2):243-255. PubMed | Google Scholar
- Englen MD, Hill AE, Dargatz DA, Ladely SR, Fedorka-Cray PJ. Prevalence and antimicrobial resistance of Campylobacter in US dairy cattle. J Appl Microbiol. 2007;102(6):1570-1577. PubMed | Google Scholar
- Papavasileiou E, Voyatzi A, Papavasileiou K, Makri A, Andrianopoulou I, Chatzipanagiotou S. Antimicrobial susceptibilities of Campylobacter jejuni isolates from hospitalized children in Athens, Greece, collected during 2004-2005. Eur J Epidemiol. 2007;22(1):77-78. PubMed | Google Scholar
- Hakkinen M, Heiska H, Hänninen M-L. Prevalence of Campylobacter spp in cattle in Finland and antimicrobial susceptibilities of bovine Campylobacter jejuni strains. Appl Environ Microbiol. 2007;73(10):3232-3238. PubMed | Google Scholar
- Miflin JK, Templeton JM, Blackall PJ. Antibiotic resistance in Campylobacter jejuni and Campylobacter coli isolated from poultry in the south-east Queensland region. J Antimicrob Chemother. 2007;59(4):775-778. PubMed | Google Scholar
- Ross CM, Donnison AM. Campylobacter jejuni inactivation in New Zealand soils. J Appl Microbiol. 2006;101(5):1188-1197. PubMed | Google Scholar
- Federighi M. How to control campylobacter in poultry farms: an overview of the main strategies. Poultry Science. 2017. Google Scholar