Is the application of Kohl to eyes associated with increased blood lead levels in children? A meta-analysis
Mina Sadeq, Touria Benamar, Antonio Facciorusso
Corresponding author: Mina Sadeq, Unit of Environmental Epidemiology, National Institute of Hygiene, Rabat, Morocco 
Received: 26 Oct 2020 - Accepted: 13 Apr 2021 - Published: 26 Apr 2021
Domain: Environmental chemistry/biology,Epidemiology
Keywords: Kohl, eye cosmetic, umbilical cord, lead, children
©Mina Sadeq et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Mina Sadeq et al. Is the application of Kohl to eyes associated with increased blood lead levels in children? A meta-analysis. PAMJ-One Health. 2021;4:17. [doi: 10.11604/pamj-oh.2021.4.17.26672]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/4/17/full
Review 
Is the application of Kohl to eyes associated with increased blood lead levels in children? A meta-analysis
Is the application of Kohl to eyes associated with increased blood lead levels in children? A meta-analysis
&Corresponding author
A controversial disagreement about the association between eye-worn Kohl and increased blood lead level (BLL) in children has been raised. We investigated such association in children aged <7 years. According to PRISMA, we performed a meta-analysis, rated the confidence in the body of human evidence, and translated the confidence ratings into levels of evidence. Detection bias related to exposure assessment, selection, and confounding were the main biases in the 14 included studies. Meta-analyses suggested a significant association between increased BLL and exposure to Kohl: odds ratio was 3.64, but heterogeneity was high (inconsistency I2=59%); pooled weighted mean difference was 5.81, but heterogeneity was high (I2=91.4%). Publication bias was ruled out in odds ratio meta-analysis. Leaving-one-out, subgroups, and meta-regression analyses showed that study quality and type of BLL testing devices were sources of heterogeneity. Biases, high heterogeneity and study size were all factors that limited evidence of the causal relationship between eye-worn Kohl and increased BLL.
Kohl, Surma, Surme, Al-kohol, Al-kahal, Kohl Al Ithmid, Asmad, Kajal, Anjana, Tiro, Tozali, or Kwalli are all names of a traditional eye cosmetic worn by adults (women and men) and children [1-7] and suspected to be responsible for increased blood lead level (BLL) causing lead poisoning [8]. In this paper, we will primarily use the term Kohl as representative of the other names. Kohl´s composition may vary. Kohl is originally obtained from a stone called Antimony or Stibium. This latter occurs chiefly as the gray sulfide mineral stibnite (Sb2S3 or antimony [III] trisulfide) [7,9]. In ancient civilizations, Antimony was well known as metal and in its sulfide form [5,10]. Due to Sb2S3 scarcity and high price over time, it was replaced by galena (i.e. lead sulfide) which is visually similar to Sb2S3. Other studies suggested that the principal constituent of Kohl from the very beginning was galena (lead sulfide) [5]. Nowadays, commercialized Kohl is available in a stone, a powder, or pencils [5,10]. Like in ancient civilizations, Kohl may be ground and used alone or mixed with other natural ingredients [5,11]. Previous studies revealed that lead contents in Kohl are highly variable, ranging from 0.00% to more than 80% [1,2,12-14]. In short, Kohl may differ by its preparation method, its added contents, and its lead concentration.
Lead (Pb) is a cumulative toxicant that has consequences on the health of both adults and children. It causes chronic diseases in adults, including cardiovascular diseases and renal impairment. Exposure of pregnant women to high lead levels can cause miscarriage, stillbirth, premature birth, and low birth weight [8]. In children, it can cause damage to the nervous system, brain and other organs at elevated BLL (i.e. 10 μg/dL or above) [15,16]. However, babies and children younger than six years are particularly vulnerable to lead poisoning [8]. They absorb 4-5 times as much ingested lead as adults from a given source [8]. Moreover, lead from Kohl may enter adults via eyes whereas mouth, eyes, and umbilical cord are primary routes of exposure to lead from Kohl in small children. Indeed, cleaning the base of newborns´ umbilical cords with oil and Kohl is common in many countries [5,17,18]; children may put their hands in their mouths after rubbing their eyes. The primary implication for health is that children who survive severe lead poisoning may be left with mental retardation and behavioral disorders [8]. Lead damage is irreversible, and its effects appear to continue to adolescence and adulthood [8]. According to the institute for health metrics and evaluation, in 2016, lead exposure accounted for 63.8% of the global burden of idiopathic developmental, intellectual disability [8]. Lead poisoning can also be fatal at very high levels in children [16]. These grounds make babies and children younger than six years an interesting, particular group to study while scoping the association between increased BLL and exposure to Kohl. The published literature gave rise to controversial disagreement as to such association [5,18]. However, to our knowledge, a meta-analysis of such association has never been performed. The objective of this study is to perform a meta-analysis and to investigate heterogeneity, potential biases, and other flaws while appraising previously published studies to answer the question of whether Kohl application to eyes is associated with an increased BLL in children aged less than 7 years, a particularly vulnerable group to lead poisoning.
This meta-analysis was performed according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines [19]. Six steps in the process were considered, including 1) specification of the research question; 2) identification of relevant studies; 3) search for and selection of studies for inclusion; 4) assessment of the quality of individual studies; 5) data extraction from studies and data analysis; 6) rating the confidence in the body of evidence.
Specification of the study question: the age range of fewer than 7 years was chosen as children of such age are very susceptible to lead exposure [20,21]; the exposure was defined as the application of Kohl to the eye; comparators were a comparison population that were not exposed (no Kohl use or occasional use); BLL was the study outcome. We put no restrictions on time or setting. Based on such PECOTS (i.e. population, exposure, comparators, outcomes, timing, and settings of interest) eligibility criteria for evaluation, a key, specific question was identified, which is "is the application of Kohl to eyes as compared to a control group associated with increased BLL (>10 μg/dL) in children aged less than 7 years?"
Search for and selection of studies for inclusion: a computerized bibliographic search was performed in PubMed, Google Scholar, Toxline, and Web of Science using both independent and combined terms, including "blood lead", "Kohl", "AlKohl", "Al-Kahal", "Surma", "eye cosmetic", "Kajal". Boolean operators were used for this purpose. We searched for studies published until August 2018; no restriction was put on location, language, subject, outcome, or study design. However, all studies had to be on humans; no age or gender restrictions were put at that stage (we excluded studies on children aged more than 7 years at the full-text assessment stage). Two independent investigators carried out the searches, and a consensus was reached by discussion. All citations were imported in Zotero, reference manager software, and duplicate citations were removed. A hand search was performed by checking the lists of references of two narrative reviews on Kohl published so far [5,18] and those of papers selected from the initial search. We also checked the references lists of two WHO documents, namely "A Review of Literature on Healthy Environments for Children in the Eastern Mediterranean Region" [16] and "Childhood Lead Poisoning" [15] for further relevant papers. Two investigators screened all titles and abstracts. A study was excluded if it was a correspondence, a letter, an editorial note, a response, a review, a paper with no original data, a study investigating Kohl content with no human data, or a study restricted to women as Kohl/Surma users.
All studies deemed relevant after the title and abstract screening were subject to full-text assessment. The study´s corresponding author or the journal was contacted to procure articles that could not be obtained. At the full-text assessment stage, we excluded a study if a control group is not identified, if data on either outcome or exposure to Kohl were not available, or if age was >7 years. Since Kohl may be applied to the umbilical cord, we also excluded neonates-related studies where the umbilical cord blood rather than venous blood was analyzed for lead levels. We included studies that measured BLL and reported mean or count to calculate odds ratio (OR). In such studies, BLL may or may not be the primary outcome, and Kohl use may or may not be the primary exposure. Also, we included study participants if they had or claimed to have a current, temporary, occasional, continuous, or past exposure to Kohl, regardless of whether lead concentration in Kohl was or was not measured. Two investigators performed the selection and identification of eligible studies; the difference in opinions was solved by discussion and following a third opinion. Information on each eligible study was collected by two investigators using a pre-established excel spreadsheet. Such information included the year of study publication, the study design, the country where the study was conducted, the setting (i.e. school, hospital or other), the study period , the age range of the study population, the exposure type (i.e. Kohl, Surma, Kajal or other), the reported route of exposure (i.e. eye only or eye & umbilical cord), the lead concentration in Kohl, the BLL threshold reported or considered by the study´s authors, the selection criteria of both cases and controls, the number of participants, the exposure assessment method (i.e. self-reporting or Kohl analysis for lead), the device used to test BLL, past or current exposure, the length of exposure, the reported risk factors of increased BLL, and the type of estimate (i.e. mean and standard deviation, count to calculate odds ratio (OR)). Should more information be needed, we contacted the corresponding author of the concerned paper.
Assessment of the quality of individual studies: following "Office of Health Assessment and Translation (OHAT) Risk of Bias (RoB) rating tool for human and animal studies" [22], we assessed the RoB corresponding to 8 domains, including selection bias, blinding, confounding bias, detection bias related to exposure assessment, detection bias related to outcome assessment, attrition bias, reporting bias and other potential bias not covered within the previously cited biases. The judgment for each domain involved assessing the risk as "Definitely high RoB", "Probably high RoB or Not reported", or "Definitely low RoB". Criteria to attribute "Definitely low RoB" were identified; a domain was rated "Definitely high RoB" if one of the related criteria was not met; "Probably high RoB or Not reported" was attributed to a domain if the required information was not reported. For selection bias, we estimated power for studies, we compared age rate in the exposed and unexposed groups as years of exposure to Kohl may affect BLL, we compared gender rate since Kohl may be applied more to female than male or vice versa [23-25], and we assessed the difference in other population characteristics (if any). Contributors to increased BLL were known to be numerous [8], Thus for confounding, we did not consider that all of them had to be controlled for in order to satisfy the criteria for low RoB: the published literature identified soil and drinking water as the significant concentrated sources of lead [26]; thus, we considered that if lead in water and soil were not measured nor controlled for, the RoB would be high. For other potential biases, we checked whether or not statistical methods were appropriate. As to rating individual studies, we reasoned that the fundamental flaws in an included study might be attributed to the following biases: exposure assessment as Pb may not be measured in individually used Kohl samples; outcome assessment since BLL may not be assessed in the same manner within groups and between groups; confounding as some studies may not consider potential risk factors related to exposure to lead from other sources, primarily soil and drinking water; the difference in characteristics between cases and controls or the lack of representativity, which may lead to selection bias. We then considered a study to be consistently high in quality if none of the following domains: detection bias, confounding bias, or selection bias was rated "Definitely high RoB". A study was rated moderate (respectively low) if only one (respectively more than one) of the three domains was rated "Definitely high RoB". Two investigators independently performed the evaluation and any difference of opinions was dealt with by re-evaluation and following a third opinion. Plots related to RoB assessments were created in RevMan (version 5.0 for Windows; the Cochrane Collaboration, Oxford, UK).
Data extraction from studies and data analysis
Data extraction: the observed counts of eligible studies were arranged into fourfold (2 by 2) tables. Exposure was identified dichotomously (i.e. Kohl users or exposed versus Kohl nonusers or unexposed). A 2 by 2 chi-square test was used to assess the association between increased BLL and exposure to Kohl in individual studies; the coefficient of contingency assessed the strength of that association. A Fisher's exact test was used as an alternative to the 2 by 2 chi-square test if the total number of observations was small. A similar analysis was performed to compare age, gender, and potential further population characteristics. Studies providing the means and standard deviation of BLL in Kohl users and nonusers were also considered. Authors of some studies were contacted to complete needed data (if any, i.e. mean or standard deviation). Estimates of BLL in μmol/L in two studies [27,28] were converted to μg/dL. To compare means in individual studies, we used the unpaired t-method that tests the null hypothesis that the population means related to two independent, random samples from an approximately normal distribution are equal. For the situation of unequal variances, Satterthwaite's approximate t-test was calculated [29]. Statistical analyses were performed using the StatsDirect statistical software version 3.0.194 (StatsDirect Ltd., Cheshire, UK). We also estimated power to detect a significant difference in frequency at the significance level of 0.05 (95% confidence level) and compared two means to detect a difference in BLL between the two groups at the significance level of 0.05 (95% confidence interval). We used OpenEpi software [30] for such analyses; a P-value was provided.
Meta-analysis: a Mantel-Haenszel test and OR at 95% confidence intervals provided a pooled OR across the strata of the fourfold table. Effect size calculations were based on weighted mean difference (wmd). The inconsistency of results across studies was summarized in the I2 statistic. A value of I2 >50% was suggestive of significant heterogeneity [31]. If so, then a random-effects model was used for the meta-analysis. Otherwise, a fixed-effect model was used. Publication bias was visually examined via a funnel plot and was statistically appreciated through the Begg-Mazumdar method based on Kendall's Tau test or Harbor method [32] if the power of the previous method was low to detect bias due to a small number of studies. In order to examine sources of heterogeneity, we first performed leave-one-out analyses to examine the influence of each study on the pooled estimate. Then, we identified a priori variables (i.e. study design, study quality, and route of exposure) and potential, exploratory variables to perform subgroup analyses and meta-regression analyses. Again, the inconsistency of results across studies was summarized in the I2 statistic. Statistical analyses were performed using the StatsDirect statistical software version 3.0.194 (StatsDirect Ltd., Cheshire, UK) and OpenMetaAnalyst software [33].
Rating the confidence in the body of evidence: to determine a confidence rating indicating the confidence with which the study findings accurately showed an actual effect of the application of Kohl to eyes on increased BLL, we followed the OHAT method [22]. Studies were given an initial rating depending on the presence/absence of four features, including "controlled exposure", "exposure before the outcome", "individual outcome data", and "comparison group use". Possible rating varies from 1 to 4 features. Such initial rating was downgraded depending on leading factors, including a risk of bias, an unexplained inconsistency of results across studies, indirectness, imprecision, and publication bias. The risk of bias was assessed via risk of bias graph, inconsistency via I2 statistic, imprecision via the width of the confidence interval, publication bias via both a Funnel plot and a Kendall's Tau test. As to indirectness, we checked if the studies assessed the population, exposure and outcome of interest. Ratings may vary from -1 (one factor) to -4 (four factors). The initial confidence rate was also upgraded depending on a dose-response gradient, residual confounding, and a significant effect. For this latter, we checked all individual eligible studies reporting an OR (wmd respectively), and we estimated that a pooled odds ratio (wmd respectively) higher than the lowest OR (wmd respectively) showing a significant association reflected a large magnitude of effect. The possible upgrading rate was +1 for each factor.
Both established downgraded and upgraded rates were combined to reveal the whole body of evidence. We followed the OHAT method [34], which used four descriptors to indicate the level of confidence in the body of evidence. They were a) high confidence in the association between exposure to the substance and the outcome, that is, the actual effect is highly likely to be reflected in the apparent relationship; b) moderate confidence in the association between exposure to the substance and the outcome, that is, the actual effect may be reflected in the apparent relationship; c) low confidence in the association between exposure to the substance and the outcome, that is, the actual effect may be different from the apparent relationship; d) very low confidence in the association between exposure to the substance and the outcome, that is, the actual effect is highly likely to be different from the apparent relationship.
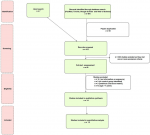
Literature search: our search procedure identified different types of articles, including original research papers, narrative review articles, short reports or letters, and case studies. Original research articles related to Kohl included those whose concern was to analyze different Kohl samples for lead and for other contents, those examining BLL in Kohl users and nonusers (i.e. BLL was the primary outcome and Kohl was the primary exposure), and those having another primary study question, but Kohl happened to be a secondary exposure. All publication types were in English, apart from a few that were in French. These were also considered and evaluated by two investigators. The search procedure yielded 464 records, from which we removed 59 duplicates. As to the hand search, most relevant papers from the different lists of references were already included in the 464 records. Thus, the hand search concerned the reference lists of 64 papers (i.e. about 14% from the initial search) and identified 7 additional potential relevant papers. Among the papers subject to full-text assessment, three papers took a few months to be obtained. One of them was not relevant, and two were eligible. A total of 14 eligible studies were included in the qualitative analysis after the removal of all abstracts and texts meeting exclusion criteria. A flow chart of the study selection procedure, based on the PRISMA statement, including identification, screening, eligibility, and included studies, was provided (Figure 1).
Characteristics of eligible studies: the 14 eligible studies were all written in English. Characteristics of such studies were shown in Table 1. Participants were babies and/or small children, recruited from a heath setting (hospital, clinic, medical center, or primary care center) in eight studies, from a community in four studies, from a school in one study. Routes of exposure were reported to be both eye and umbilical cord in two studies, only eye in twelve studies (Table 1). The name of the eye cosmetic differed in included studies. It was primarily Kohl in the Middle East, Surma and Kajal in India and Pakistan (Table 1). The primary study question was related to BLL/lead poisoning and its potential predictors in 8 studies (Kohl happened to be a secondary exposure), it was about association between BLL and Kohl use in 5 studies (Kohl was the primary exposure), and about link between BLL and feeding practices in one study. Thus, BLL was the main, unique outcome in all 14 eligible studies, whereas Kohl was the primary exposure in five studies [27,28,35-37], a secondary exposure in nine studies [17,25,38-44]. Except for one [36], all studies were observational, cross-sectional studies; however, it is worthy to note that the study subject to an exception was a retrospective chart review whose aim was to compare BLL in Pakistani/Indian children using leaded-eye cosmetics and those not using such products, and for which sufficient data were available to compare such groups [36]. Such a study had characteristics similar to those of a cross-sectional study (i.e. data on exposure and outcome were collected at the same time, it is not known if exposure preceded the outcome or vice versa; a sample of children was selected, it was further subdivided into exposed and unexposed to leaded-eye cosmetics, and BLL in such subgroups were compared). It was then assessed as such and combined with the other cross-sectional studies in the meta-analysis (Table 1). Exposure to Kohl was self-reported. The lead concentration in Kohl was measured only in 4 studies [27,28,36,40], but it was not individually or representatively assessed. Moreover, there was insufficient information about exposure duration and frequency.
Comparators were reported as "no Kohl users" in 11 studies, "participants not receiving Kohl for at least two months before sampling" in one study, "children that have never used Kohl or have used it occasionally" in one study. Various BLL testing devices were reported, including inductively coupled plasma mass spectrometry in one study [42], atomic absorption spectrophotometer (AAS) in 11 studies, a lead-care analyzer instrument in 2 studies [25,38], and a portable X-ray fluorescence spectrometer in 1 study [41]. BLL ≥10 ug/dL was reported as a cut-off point in 13 eligible studies. The remaining eligible study reported dichotomous outcome data; in such studies, BLL ≥5 ug/dL was the cut-off point [41]. It could thus not be included in the meta-analyses. Of the 14 studies, three studies [27,42,43] reported both dichotomous outcome data (i.e. counts to calculate OR) and mean outcome data as statistical estimates, five studies reported dichotomous outcome data only [17,34,36,37,39], and six studies outcome mean data only [25,28,35,38,41]. We requested information/data from the authors of four papers, but authors of only one paper responded.
Potential reported covariates were age, gender, caste (social class in Hindu society), religion, socio-economic status, parental occupation, lead from the soil, lead from drinking water, location next to a road, PICA behavior, painted houses, thumb-sucking, painted toys, eating from outside vendors, use of herbal medicine, sindoor (a traditional red cosmetic usually worn by married women along the part of their hair) use and breastfeeding; a varying number of controlled covariates was shown across included studies. Multivariable analysis was performed in most studies; however, soil and drinking water were not analyzed for lead in several of them. Furthermore, sufficient data on such predictors were not available for all the 14 included studies to be considered in the meta-analysis. Power calculation indicated that all studies reporting dichotomous outcome data had a low power (<80%) except for two [17,43] and that all studies reporting mean outcome data had a high power (>80%) except for one [25]. We rated the eight domains of bias in individual studies. Both the overall risk of bias and a risk of bias summary were shown in Figure 2. Selection bias, confounding, and exposure assessment were important, prevailing biases in most of the included studies (Figure 2); their respective proportion was >25%, >60%, and 100%. Four studies were classified as moderate quality [25,38,43,44]; the remaining ten studies were considered low quality.
Estimation of the pooled OR: meta-analysis of pooled OR involved seven studies (Figure 3) and included a total of 1239 children aged between 4 months and seven years (i.e. 364 Kohl users versus 875 nonusers). A significant association was shown between increased BLL and exposure to eye-worn Kohl (Figure 3) with a pooled OR=3.64 (95% CI=(1.81 - 7.46), P=0.0003), however, heterogeneity was high (I2 = 59%; Cochran Q = 14.56 with P = 0.024). Publication bias was ruled out (P>0.3).
Sensitivity analysis: two studies, [36,38], had high estimates (30.3 and 9.1 respectively). Leaving the first one out decreased estimate to 2.71 and lowed heterogeneity (I2 = 0%; Cochran Q = 4.72 with P = 0.451; publication bias was ruled out P>0.5), whereas leaving the second one out did not change effect estimate or heterogeneity. For the remaining studies, the leaving-one-out analysis showed a relatively stable estimate (3.74 - 4.24) and only insignificant variations in heterogeneity. Besides study quality and route of exposure, we identified exploratory variables to further examine sources of heterogeneity. Those included type of BLL testing devices (i.e. AAS versus other devices), study question (i.e. Kohl as a primary exposure in a study versus Kohl as a secondary exposure), and the recruitment location of the participant (i.e. a health service versus a community/school). As all studies included in the meta-analysis were cross-sectional studies, we also wondered whether the rate of users/nonusers (i.e. if a study included more Kohl users than nonusers or vice versa) was a source of heterogeneity. Separate stratification by study quality and by route of exposure gave a different OR in compared strata, whereas separate stratification by study question and by rate of users/nonusers gave a relatively similar OR in compared strata. Stratification by type of BLL testing device was not possible as inductively coupled plasma mass spectrometry was used only in one study (OR = 1.03; P>0.05) [42], lead care analyzer instrument in only one study (OR = 30.30; P<0.0001) [38]; the remaining five studies used AAS and gave an OR of 3.12 (95% CI = (1.96 - 4.97), P<0.0001) with no heterogeneity. Heterogeneity was high in all the strata that included the study [38] and low in the strata that did not include that study, and this was consistent with leave-one-out meta-analyses. The meta-regression analysis required a choice of the variables to be included in the statistical model. The idea was to include in the model the previously identified a priori variables (i.e. study quality and route of exposure) and other potential exploratory variables. So, we did not consider the study question, the rate of users/nonusers, or the recruitment location of the participant as heterogeneity became low (P Cochran >0.05) when we removed the study [38] from the strata showing high heterogeneity. Also, since that study differed from the other studies in terms of the type of device, we included that variable in the model. Results of meta-regression analysis revealed that the type of BLL testing device was a significant contributor to heterogeneity (Table 2), and this was consistent with the other sensitivity analyses. The study [38], in particular, used a lead-care analyzer instrument. The significant OR was verified in all sensitivity analyses (P<0.05).
Estimating the mean difference of BLL between Kohl users and nonusers: effect size meta-analysis combined the results of seven studies (Figure 3), included 1565 children (350 users versus 1215 nonusers) aged less than 7 years, and resulted a significant pooled wmd of 5.81 (95% CI = (2.17 - 9.45); P = 0.002). However heterogeneity was high (I2 = 91.4%; Cochran Q = 69.70 with P < 0.0001). Publication bias was observed (P = 0.03).
Sensitivity analysis: the study [27] had the highest estimate. Omitting that study did not change the magnitude of the association or the heterogeneity. Such a similar result was found in the remaining studies, except for the study [25]. Omitting that study from the meta-analysis increased the magnitude of the association (pooled effect size wmd = 6.68 (95% CI = 5.31 - 8.05) and reduced heterogeneity to 53.8%; Cohran Q = 10.82 with P = 0.06; publication bias was ruled out P>0.6). Stratification by type of testing device was not possible as only one study, [25], used a lead care analyzer instrument. Separate stratification by study quality, study question, and recruitment location of the participant gave a different estimate in compared strata. The significant wms was not confirmed in moderate quality studies, in studies where Kohl use happened to be a secondary exposure, and in studies on participants recruited from a community/school. In meta-regression analysis, we did not consider exploratory variables such as the study question, the rate, or the recruitment location of the participant since heterogeneity disappeared when we removed the study [25] from the strata showing high heterogeneity. We included study quality (as a priori variable) and type of device in the statistical model. The results of the meta-analysis identified study quality as a contributor to heterogeneity (Table 2).
Rating the confidence in the body of evidence: in all relevant studies, the outcome was assessed at the individual level, and a comparison group was used within the study, which gave an initial confidence rate of "moderate". Such rate was downgraded by -4, upgraded by +1. We rated the overall quality of the human evidence "very low". A summary of quality of evidence for Kohl's application to humans' eyes was provided in Table 3.
Interpretation of the findings: in this work, we rigorously dealt with potential biases while appraising previously published studies to answer the question whether Kohl's application to the eyes was associated with an increased BLL in children aged less than seven years. The overall quality of the human evidence was rated as "very low". This result revealed that further research was very likely to influence confidence in the apparent relationship between eye-worn Kohl and increased BLL. Statistical probability endorsed such findings; thus, even if an association was observed between increased BLL and Kohl application to the eyes, confidence in such association was limited by the presence of various significant biases. Exposure assessment was the main issue. Kohl may contain from 0.00% to more than 80% of lead [1,2,12-14], it may be sold in tiny containers, and one may use one single Kohl container or interchangeable containers. All these factors may make it challenging to quantify exposure to lead from Kohl. Our results showed that data on lead concentration in Kohl at the individual level was limited. So were data on frequency, duration of exposure, current or past exposure. The exposure was based on the self-reporting use of Kohl. Therefore, detection bias and potential misclassification could not be ruled out. Moreover, our results showed that studies reporting the application of Kohl to the eye and umbilical cord had higher effect estimates than studies reporting eyes as a single route of exposure, which may suggest combined effects of the two sources of exposure on BLL. However, when considering only moderate-quality studies’ reporting eye-worn Kohl, the mean difference was not significant, and evidence of heterogeneity was low. These findings may imply that Kohl's application to the umbilical cord rather than to the eyes may raise BLL. Further future well-designed studies would better advice on this.
Confounding was a further issue. In most of the studies, confounders, particularly soil and drinking water, were not controlled for; more importantly, not all controlled covariates confirmed a negative effect. More surprisingly, all reported BLL means in Kohl nonusers exceeded 10 μg/dL; even though two studies, [35,42], were exceptions, they still raised questions. Nir et al. (1992) reported that Kohl nonusers were identified as babies that have received Kohl in their past lives; however, even though lead was known to be a cumulative toxicant, the mean BLL in such babies was less than 5 μg/dL (4.3 μg/dL) [35]. Furthermore, in the other study [42], the mean BLL in Kohl nonusers was higher (8.1 μg/dL) than that in Kohl users (5.2 μg/dL). The additional contribution of confounders to produce a combined effect may explain such findings. Selection bias was an additional concern. In studies where Kohl was not the primary exposure, characteristics (age, gender, and others) of Kohl users and nonusers were not reported. Lack of data on such characteristics, low participation rate, and low study power may have increased the risk of bias and distorted the results. The study question, the recruitment location of participants, and the analytical accuracy and precision should all be considered when conducting a study on the effect of Kohl on increased BLL.
Our meta-analysis has limitations and strengths. There were limitations inherent in the available literature. For example, due to limited studies advising on lead content in Kohl at the individual level, we could not explore the dose-response relationship. Such information would have upgraded evidence. Also, we could not examine the 5 μg/dL threshold value since only one study considered such value. Moreover, the cross-sectional design of the studies included in our meta-analysis may raise the question of whether increased BLL occurred before exposure to Kohl. In those studies, the mean BLL in Kohl nonusers generally exceeded 10 μg/dL, and this may point to such possibility. However, would be the case, it may support our finding. Furthermore, our meta-analysis concerned only children from East countries where commercial, black, high-lead-Kohl from India may prevail. Such analysis did not include children from North Africa where the preparation method of Kohl, its added contents, and its lead level may differ. This was due to limited studies on children exposure to Kohl in that latter area. The intention is to encourage epidemiological relevant studies in North Africa. Despite all these limitations, this was the first meta-analysis about increased BLL from Kohl application to the eyes so far. The comprehensive search of papers related to Kohl, the systematic search of the possible risk of bias, and the thorough search of possible sources of heterogeneity that were examined via various sensitivity analyses (such as leave-one-out analysis, subgroup analysis, and meta-regression) were all further strengths of our work.
Biases, high heterogeneity, and study size were all factors that limited evidence of the causal relationship between eye-worn Kohl and BLL increase. Further research efforts should be placed on studies with better exposure assessment and better controls of potential confounders. A good choice of device testing for BLL is also advised.
What is known about this topic
- Kohl is a traditional eye cosmetic worn by children and suspected to be responsible for increased blood lead level (BLL);
- The published literature gave rise to controversial disagreement as to such association;
- A meta-analysis of such association has never been performed.
What this study adds
- A comprehensive search of papers related to Kohl, a systematic search of the possible risk of bias, and a meticulous search of possible sources of heterogeneity via various sensitivity analyses such as leave-one-out analysis, subgroup analysis, and meta-regression were all performed;
- Biases, high heterogeneity, and study size were all factors that limited evidence of a causal relationship between eye-worn Kohl and BLL increase;
- • Further research efforts should be placed on studies with better exposure assessment and better controls of potential confounders.
The authors declare no competing interests.
All authors contributed to this paper. MS conceived the study, performed the statistical analysis and wrote the paper. MS, TB, AF contributed to papers search and quality assessment, and rated the confidence in the body of evidence. All the authors have read and agreed to the final manuscript.
We also would like to acknowledge the authors of selected papers for their assistance on completing needed information relevant to their published papers.
Table 1: characteristics of studies on high BLL and exposure to Kohl (14 human observational studies)
Table 2: meta-regression analysis of heterogeneity
Table 3: criteria for evaluating the quality of the body of human evidence
Figure 1: flowchart summarizing study selection, this is a PRISMA flow diagram that shows the different steps of the systematic review and the number of studies identified, screened, included and excluded
Figure 2: risk of bias, this figure shows each risk of bias item presented as percentages across all included studies (in right) and risk of bias item for each included study (in left)
Figure 3: forest plot, this figure shows the studies providing an odds ratio (left) and a weighted mean difference (right) of the association between exposure to eye-worn Kohl and increased BLL in children; random effect model found a significant OR for combined studies, with high heterogeneity; random effect model found a significant wmd for combined studies, with high heterogeneity
- Al-Kaff A, Al-Rajhi A, Tabbara K, El-Yazigi A. Kohl - the traditional eyeliner: use and analysis. Ann Saudi Med. 1993;13(1):26-30. PubMed | Google Scholar
- Andalib S, Rizwani GH, Sharif H, Arman M. Chemical and toxicological studies on different brands of Asmad (Antimony sulphide) available in Pakistan and Saudi Arabia. Pak J Pharm Sci. 2018;31(6):2591-5. PubMed | Google Scholar
- Badeeb OM, Ajlan RS, Walid MH. Kohl Al-Ethmed. JKAU Med Sci. 2008;15(4):59-67. Google Scholar
- De Caluwé JP. Lead poisoning caused by prolonged use of Kohl, an underestimated cause in French-speaking countries. J Fr Ophtalmol. 2009;32(7):459-63. PubMed | Google Scholar
- Mahmood ZA, Zoha SMS, Usmanghani K, Hasan MM, Ali O, Jahan S et al. Kohl (surma): retrospect and prospect. Pak J Pharm Sci. 2009;22(1):107-22. PubMed | Google Scholar
- Neelotpol S, Hia RA. Lead exposure of Bangladeshi women at childbearing age: does mother´s education reduce fetal risk factors. J Local Glob Health Sci. 2016;1. Google Scholar
- Sweha F. Kohl along history in medicine and cosmetics. Hist Sci Medicales. 1982;17(Spec 2):182-3. PubMed | Google Scholar
- WHO. Lead poisoning and health. 2018. Accessed 8 April 2019.
- Shortland AJ. Application of lead isotope analysis to a wide range of late Bronze age Egyptian materials. Archaeometry. 2006;48(4):657-69. Google Scholar
- Pasha MA. X-ray Study of Kohl stone used in Indian traditional medicine and cosmetics. IJEE. 2016;2(1):1-5.
- Al-Ashban RM, Aslam M, Shah A. Kohl (surma): a toxic traditional eye cosmetic study in Saudi Arabia. Public Health. 2004 Jun;118(4):292-8. PubMed | Google Scholar
- Aslam M, Davis SS, Healy MA. Heavy metals in some Asian medicines and cosmetics. Public Health. 1979;93(5):274-84. PubMed | Google Scholar
- Haq I, Khan C. Hazards of a traditional eye-cosmetic-SURMA. JPMA J Pak Med Assoc. 1982;32(1):7-8. PubMed | Google Scholar
- Madany IM, Akhter MS. Lead levels in some eye cosmetics used in Bahrain. J Environ Sci Health Part Environ Sci Eng Toxicol. 1992;27(6):1541-7. Google Scholar
- WHO. Childhood lead poisoning. 2010. Accessed April 5 2019.
- WHO Regional Office for the Eastern Mediterranean. A review of literature on healthy environments for children in the Eastern Mediterranean Region I: status of childhood lead exposure. 2005. Accessed April 1 2019.
- Nuwayhid I, Nabulsi M, Muwakkit S, Kouzi S, Salem G, Mikati M et al. Blood lead concentrations in 1-3 year old Lebanese children: a cross-sectional study. Environ Health Glob Access Sci Source. 2003;2(1):5. PubMed | Google Scholar
- Tiffany-Castiglioni E, Barhoumi R, Mouneimne Y. Kohl and surma eye cosmetics as significant sources of lead (Pb) exposure. J Local Glob Health Sci. 2012;1. Google Scholar
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-9, W64. PubMed | Google Scholar
- Chiodo LM, Covington C, Sokol RJ, Hannigan JH, Jannise J, Ager J et al. Blood lead levels and specific attention effects in young children. Neurotoxicol Teratol. 2007;29(5):538-46. PubMed | Google Scholar
- Parry C, Eaton J. Kohl: a lead-hazardous eye makeup from the third world to the first world. Environ Health Perspect. 1991;94:121-3. PubMed | Google Scholar
- OHAT Risk of Bias Tool. OHAT risk of bias rating tool for human and animal Studies. OHAT. 2015;37.
- Al-Saleh I, Nester M, DeVol E, Shinwari N, Al-Shahria S. Determinants of blood lead levels in Saudi Arabian schoolgirls. Int J Occup Environ Health. 1999;5(2):107-14. PubMed | Google Scholar
- Kaufman JA, Brown MJ, Umar-Tsafe NT, Adbullahi MB, Getso KI, Kaita IM et al. Prevalence and risk factors of elevated blood lead in children in gold ore processing communities, Zamfara, Nigeria, 2012. J Health Pollut. 2016;6(11):2-8. PubMed | Google Scholar
- Roy A, Hu H, Bellinger DC, Palaniapan K, Wright RO, Schwartz J et al. Predictors of blood lead in children in Chennai, India (2005-2006). Int J Occup Environ Health. 2009;15(4):351-9. PubMed | Google Scholar
- Maru S. Lead exposure in children through water and soil. Environ Manag Risk Assess PH 560. 2015;16. Google Scholar
- Ali AR, Smales OR, Aslam M. Surma and lead poisoning. Br Med J. 1978;2(6142):915-6. PubMed | Google Scholar
- Green SD, Lealman GT, Aslam M, Davies SS. Surma and blood lead concentrations. Public Health. 1979;93(6):371-6. PubMed | Google Scholar
- Armitage P, Berry G, Matthews JNS. Statistical methods in medical research 4th edition; Malden, MA. Wiley-Blackwell. 2001.
- Dean A, Sullivan K, Soe M. OpenEpi open source epidemiologic statistics for public health. Accessed 5 April 2019.
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-60. PubMed | Google Scholar
- Harbord RM, Egger M, Sterne JAC. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443-57. PubMed | Google Scholar
- Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1-15. Google Scholar
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122(7):711-8. PubMed | Google Scholar
- Nir A, Tamir A, Zelnik N, Iancu TC. Is eye cosmetic a source of lead poisoning. Isr J Med Sci. 1992;28(7):417-21. PubMed | Google Scholar
- Sprinkle RV. Leaded eye cosmetics: a cultural cause of elevated lead levels in children. J Fam Pract. 1995;40(4):358-62. PubMed | Google Scholar
- Malik Q, Hafeez A. Effects of surma use and pica on blood Lead levels of preschool children. Pakistan Armed Forces Medical Journal. 1999;49(1):18-20.
- Al- Khateeb AR, Ahmed WG, Ahmed MM, Al-Jawadi AA, Al- Naemi AH. Blood lead levels in 1-5 years old children in Mosul, Iraq: a cross - sectional study. J Bahrain Med Soc. 2007;19:100-6.
- Boseila SA, Gabr AA, Hakim IA. Blood lead levels in Egyptian children: influence of social and environmental factors. Am J Public Health. 2004;94(1):47-9. PubMed | Google Scholar
- Pfitzner MA, Thacher T, Pettifor J, Zoakah A, Lawson J, Fischer PR. Prevalence of elevated blood lead in Nigerian children. Ambul Child Health. 2000;6(2):115-23. Google Scholar
- Getso KI, Hadejia IS, Sabitu K, Nguku PM, Poggensee G, Muhammad Aliyu H et al. Prevalence and determinants of childhood lead poisoning in Zamfara State, Nigeria. J Health Pollut. 2014;4(6):1-9. Google Scholar
- Panahandeh G, Khoshdel A, Heidarian E, Amiri M, Rahiminam H. Blood Lead Levels in children of Southwest Iran, aged 2-6 years and associated factors. J Clin Diagn Res JCDR. 2017;11(7):SC01-4. PubMed | Google Scholar
- Patel A, Williams SV, Frumkin H, Kondawar VK, Glick H, Ganju AK. Blood Lead in children and its determinants in Nagpur, India. Int J Occup Environ Health. 2001;7(2):119-26. PubMed | Google Scholar
- Patel AB, Belsare H, Banerjee A. Feeding practices and blood lead levels in infants in Nagpur, India. Int J Occup Environ Health. 2011;17(1):24-30. PubMed | Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
 Figure 3: forest plot, this figure shows the studies providing an odds ratio (left) and a weighted mean difference (right) of the association between exposure to eye-worn kohl and increased BLL in children; random effect model found a significant OR for combined studies, with high heterogeneity; random effect model found a significant wmd for combined studies, with high heterogeneity
Figure 3: forest plot, this figure shows the studies providing an odds ratio (left) and a weighted mean difference (right) of the association between exposure to eye-worn kohl and increased BLL in children; random effect model found a significant OR for combined studies, with high heterogeneity; random effect model found a significant wmd for combined studies, with high heterogeneity