Magnitude and trends of ruminants, Pigs and poultry diseases in Taraba State, Nigeria, 2013- 2017: implications for public health
Ayi Vandi Kwaghe, Daniel Egom Okomah, Mabel Kamweli Aworh, Emmanuel Awosanya, Chukwuma David Umeokonkwo, Emmanuel Yarai, Celestine Ameh, Junaid Kabir
Corresponding author: Ayi Vandi Kwaghe, Department of Veterinary and Pest Control Services, Federal Ministry of Agriculture and Rural Development, Abuja, Nigeria 
Received: 29 Jun 2020 - Accepted: 07 Aug 2020 - Published: 11 Aug 2020
Domain: Public health
Keywords: Antimicrobial resistance, livestock, one health approach, transboundary animal diseases, zoonoses
©Ayi Vandi Kwaghe et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Ayi Vandi Kwaghe et al. Magnitude and trends of ruminants, Pigs and poultry diseases in Taraba State, Nigeria, 2013- 2017: implications for public health. PAMJ-One Health. 2020;2:20. [doi: 10.11604/pamj-oh.2020.2.20.24652]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/2/20/full
Research 
Magnitude and trends of ruminants, Pigs and poultry diseases in Taraba State, Nigeria, 2013- 2017: implications for public health
Magnitude and trends of ruminants, Pigs and poultry diseases in Taraba State, Nigeria, 2013- 2017: implications for public health
Ayi Vandi Kwaghe1,2,3,&, Daniel Egom Okomah1,2, Mabel Kamweli Aworh1,2, Emmanuel Awosanya4, Chukwuma David Umeokonkwo2,5, Emmanuel Yarai1, Celestine Ameh2, Junaid Kabir6
&Corresponding author
Introduction: livestock diseases could pose a threat to public health through zoonoses and exacerbation of antimicrobial resistance with indiscriminate drug use. Understanding and managing public health threats at the human-animal-environment interface is key to global health security. We determined the magnitude and trends of zoonotic and Transboundary animal diseases (TADs) among livestock in Taraba State, a nexus for livestock activities in Nigeria.
Methods: we reviewed records of clinical cases between 2013 and 2017 at the Veterinary Services Department, Ministry of Agriculture and Natural Resources, Taraba State. Data on livestock species, clinical diagnosis and sex were extracted and analyzed using descriptive statistics.
Results: of the total 1,535,267 cases in ruminants and pigs, top zoonotic diseases were Helminthoses (43.0 %), Trypanosomosis (21.2%) and ectoparasitism (14.9%); while TADs were Peste des Petits Ruminants (2.1%), Contagious Bovine Pleuropneumonia (1.4%) and Foot and mouth disease (1.3%). Cumulatively, 87.3% of the cases in ruminants and pigs in Taraba State were zoonotic diseases. Of the total 237,671 cases in poultry, the most reported was coccidiosis (71.4%); disease of zoonotic importance was enteric salmonellosis (3.1%), and the most TAD was Newcastle Disease (8.4 %). More than half (53.9 %) of the ruminant and pig cases were females. The frequency of occurrence of most cases was regular across the years.
Conclusion: the magnitude and pattern of animal diseases of zoonotic and public Health importance in Taraba State is high and endemic. Public education and One-Health approach involving veterinary services; public and environmental health is advocated towards the prevention and control of these diseases.
Zoonoses are diseases naturally transmitted between animals and humans, posing a great threat to the health and life of people all over the world [1]. World Bank estimate of zoonotic disease outbreaks worldwide for a decade cost 200 billion USD as a result of loses due to trade, tourism and tax revenues [2]. Presently, over 200 pathogens are regarded as zoonoses. The WHO in 2015 indicated that almost 600 million cases of diseases were caused by contaminated food in 2010 and about 350 million caused by pathogenic bacteria [3]. In the EU, there are over 200,000 cases of bacterial zoonoses noted annually with presumably much higher numbers of real cases. According to the European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC), the most common causes of food-borne zoonotic diseases were Campylobacter and Salmonella bacteria [4]. The occurrence of these pathogens is more likely where there is poor surveillance, low level of biosecurity and hygiene in production and processing. Developing countries, particularly those in sub-Saharan Africa, are enormously challenged by infectious diseases of animals that are, in most instances, transboundary and or zoonotic in nature. The presence of these diseases further exacerbates the endemic poor productivity of livestock, food insecurity and low food safety standards. Zoonotic diseases also add to health care burden further reducing the benefits derivable from animal companionship and production.
The food and Agricultural Organization of the United Nations postulated that the livestock subsector is expected to grow significantly and transform in the next 30 to 40 years [5]. Tendencies are increase in zoonoses if proactive measures are not taken to prevent and control these diseases in animal population. In 2050, the population of Nigeria will reach about 400 million, from 180 million people today; 280 million people will live in urban areas compared to the current 90 million people [5]. This will lead to increase interaction between the animal-human-environment interface, hence the need to protect public health, increase food safety and food security of the nation with regards to the FAO goal towards a sustainable livestock [5]. Part of the response to increased demand for food is intensification of agriculture including livestock and poultry production. This will likely necessitate use of extra chemical inputs including antibiotics, that are already overused particularly in the management of infectious diseases such as Peste des Petit Ruminants (PPR) Contagious Bovine Pleuropneumonia (CBPP), Food and Mouth Disease (FMD) and New Castle Disease. Antimicrobial resistance is a priority threat to global public health.
Taraba state is a border state located in the North-Eastern part of Nigeria and one of the important entry points into the country. Three out of the seven international control posts in the eastern part of the country are located in Taraba. It is a hub for livestock activities with a significant number of nomadic settlements. Taraba state projected livestock population for the 2018 was 23,549,584 million; 5,577,980 cattle, 3,061,666 sheep, 3,686,973 goats, 3,212,979 pigs and 8,009,986 poultry. The huge presence of livestock activities in the state and the potential spillover of zoonotic diseases and antimicrobial resistance in human population necessitated the need to intensify animal disease surveillance in the state. The National Animal Disease Information System (NADIS) captures data from routine passive disease reports, these reports are hardly utilized for the control of zoonotic diseases in the country, because, while NADIS is resident in the Federal Ministry of Agriculture and Rural Development, human diseases are captured by the Integrated Disease Surveillance and Response (IDSR) system in the Federal Ministry of Health. The two systems are functionally unconnected. Thus, data on zoonotic diseases remain under-utilized for control and prevention purposes. This study aimed at determining the magnitude and trend of animal diseases; proportions of the diseases that are zoonotic and TADs based on species and sex as well as discuss their public health implications in Taraba State.
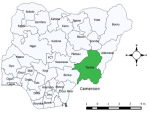
Study area: Taraba State is situated in the north eastern part of Nigeria between latitude 6° 30´ and 8° 30´ north of the equator and between longitude 9° 00´ and 12° 00´ east of the Greenwich meridian. Taraba State shares border with six states in Nigeria; in the west by Nasarawa and Benue States, northwest by Plateau State, north by Bauchi and Gombe States, Adamawa State on the north-eastern part and Cameroon on the east and southern part of the State (Figure 1). The state has a land area of 60,291km2 with a population of about 2.5 million people projected from the 2006 National Population Census [6]. Occupation of the Taraba State people is mainly agriculture. The Mambilla Plateau is a unique agricultural site where large numbers of ruminants are reared. Other common sites where livestock are reared in the state include the Benue and Taraba valleys. Aside ruminants, other livestock production activity in the state includes poultry production, rabbit breeding and pig farming [7].
Data collation and analyses: a retrospective study was carried out on clinical cases of animal diseases reported to the Taraba State Ministry of Agriculture between 2013 and 2017. A total of 10 veterinary health facilities (eight clinics and two hospitals) report on monthly basis to the State Epidemiology Officer, Department of Veterinary Services, Ministry of Agriculture and Natural Resources, Taraba State. Out of the eight clinics, six were public while two were private. The collated veterinary records (N = 1,772,938) were reviewed and data on species of animal affected, sex and clinical diagnosis were extracted. Diagnosis were based on signs, symptoms and postmortem examination where applicable. Data were imputed into Microsoft Excel version 2010 and analyzed using descriptive statistics.
A total of 1,535,267 cases in ruminants and pigs were reported for the five-year period under review. Of the total cases in ruminants and pigs, 1,239,677 (80.4%), 171,295 (11.1%), 119,154 (7.7%), and 12,306 (0.8%) were reported in cattle, goats, sheep and pigs, respectively. Overall, more of the reported cases were in female (53.9%) (Table 1). In general, the most reported case was helminthoses (43.0%), while the least was cowdriosis (0.04%). The most reported case with zoonotic potential was also helminthoses (43.0%), then trypanosomosis (21.2%); the least reported being salmonellosis (0.4%). The number of cases of fasciolosis reported was 4.8% (31837/659999) compared to the entire cases of helminthoses. TADs were PPR (2.1%), while the least reported was FMD (1.3%); Table 2. Of the total 237,671 cases in poultry within the five-year period, the most reported case was coccidiosis (71.4%), while the least reported case was Gumboro disease (1.6%); disease of zoonotic importance was enteric salmonellosis (3.1%), and the most TAD was Newcastle Disease (8.4%) (Table 3). The number of cases reported in the five-year period under review seem to oscillate, but regular in occurrence (Figure 2).
Food borne zoonoses that were identified from this study were helminthoses and Salmonellosis. Helminthoses ranked the highest among zoonotic cases for the period of five years. Helminthoses is a disease of public health importance with transmission potential from ruminants and pigs to humans through the consumption of improperly cooked meat. This stresses the need for genuine sensitization of livestock farmers on routine deworming of their animals to guarantee the supply of hygienic meat to the public. Data obtained also highlights the relevance of taking the health of the food animals seriously to secure the health of the public. The study indicates cattle constituting great proportion of reported cases of helminthoses. Which is a pointer that more effort need to be targeted toward this species in the prevention and control programme that should be implemented. Also, most of our cattle in the country are under extensive management system and the cattle end up with rivers and streams as their major source of drinking water [5] culminating in infection with helminths during grazing on contaminated pasture by other animals that are the definitive hosts especially during the rainy season. The management system of our cattle need to be modified in such a way that clean drinking water is made available for the animals. Cases of faciolosis constituted 4.8% of the cases of helminth infection. Fasciola hepatica and Fasciola gigantica are the major causative agents of liver fluke disease (fasciolosis) in domestic animals in temperate and tropical climates [8]. Fasciolosis is an important emerging zoonotic disease of humans and is endemic in Nigeria [9]. Transmission occurs where rural farming communities share the same water source with their animals or consume water-based vegetation growing in endemic areas [9]. Fasciola hepatica can survive for up to 13 years in humans, producing large numbers of eggs (up to 5000 per gram of faeces) that are infective to other hosts, humans are also likely to play an important role in transmission of the disease [10]. Hyperendemic human fasciolosis has also been reported in the Nile Delta region between Cairo and Alexandria [11].
A number of studies in Nigeria have demonstrated the presence of zoonotic helminths, one of which revealed high prevalence of zoonotic helminth infection among humans (7.8%) and animals (18.1%) in Jos, Plateau state [12]. In another study, the prevalence and geographical distribution of zoonotic helminths in food animals slaughtered in Nigerian abattoirs between 1970 and 2016 across 19 Nigerian states was 7.48% (ranging from 1.90% to 60.98% across strata) with highest prevalence estimates in the north-central region of Nigeria [13] where Taraba State is located. This report is in agreement with our findings in this study, with helminthoses having the highest occurrence among the clinical cases in the state, emphasizing the threat of zoonotic helminthoses to both animal and human populations in the state. Globally, the risk of zoonotic helminthoses is increasing [14,15] in Africa and Asia. Cysticercosis is fast-becoming an emerging zoonosis in some developed countries, notably the United States of America (USA) [16] despite the fact that it is considered to be an eradicable disease [17] by means of early diagnosis [18,19] and treatment. Salmonellosis (0.41 %) was clinically diagnosed in ruminants and pigs. Studies by [20], in Maiduguri indicated the presence of Salmonella specie in fresh meat of cattle, goat, sheep and camel (beef, chevon, mutton and jaziir) in samples that were obtained from the abattoir, market (meat retailers) and meat shops with a recorded prevalence of 26.7%. Report indicate the presence of Salmonella from stool samples of humans and cattle in Lagos state, Nigeria [21]. Study by [22], from Ethiopia indicated the presence of Salmonella in the samples collected from apparently healthy sheep and goats with a prevalence of 1.04% from the 384 animals that were tested. Vector borne diseases obtained from this study were Trypanosomosis, Babesiosis and Anaplasmosis. Trypanosomosis which is transmitted by Tsetse flies which covers approximately 80% of the landmass in Nigeria [23]. Hence, the continuous thriving of African Animal Trypnosomosis (AAT) and incurred losses. This study revealed trypanosomosis as a problem in the livestock subsector in Taraba state constituting about (21.2%) of the total cases that were reported thereby making the human populace vulnerable to the disease. A study in Nigeria reported a high overall prevalence of 16.1% of AAT [24]. Another study conducted in Plateau state revealed a high prevalence (46.8 %) of bovine trypanosomiasis (Trypanosoma brucei brucei, Trypanosoma congolense savannah, Trypanosoma vivax) [25]. The high prevalence (46.8 %) of bovine trypanosomosis detected in Plateau should be a source of concern to Taraba state as it shares border with Plateau state couple with the already high proportion of cases of trypanosomosis in the state. There are high chances of transmission AAT to the human populace in Taraba state.
Ectoparasites were also among the major problems that ruminants face in Taraba state based on this study. Ranking third among the clinical cases in the state and remained constantly high through the years examined except for year 2014 where the prevalence was 2.78%. An extensive review of reports tagged “tick, flea, and louse-borne diseases of public health and veterinary significance in Nigeria” [26], conducted across the nation revealed the presence of diverse species of ectoparasites in the country. This further explains the diagnosis of Babesiosis and Anaplasmosis in animals in the state due to the presence of ticks. Quite a number of reports around the nation has demonstrated numbers of species of ticks and implicated them in a lot of disease conditions [27-32]. Ectoparasites are among the major problems that ruminants face in Taraba State based on information obtained. This further explains the diagnosis of Babesiosis and Anaplasmosis as tick borne diseases of animals in the state. An extensive review of reports tagged “tick, flea, and louse-borne diseases of public health and veterinary significance in Nigeria” [26], conducted across the nation demonstrated quite a number of species of ticks and implicated them in a lot of disease conditions [27-29,31,32]. The presence of these ticks is also a threat for transmission of diseases to the human populace. Perhaps, Babesiosis and Anaplasmosis are currently circulating within the human population without being diagnosed. There is need for research to be conducted in humans to confirm the presence of Babesiosis and Anaplasmosis in Taraba state. A study in Bangladesh [33] determined the prevalence of ectoparasites infestation of cattle in and around the Bhawal forest area in Gazipur district found 132 (64.07%) of 206 cattle to be infested with several species of ticks and lice. The study also revealed significantly higher prevalence of ectoparasites infestation in cattle reared under free range system than that of semi-intensive system [33]. Reasons for high tick infestation includes free range management system, malnourished cattle with poor health status [33]. The endemicity of ectoparasites and its high ranking among cases presented in the state indicates the need for strategic prevention and control of ectoparasites in the environment where animals are kept and treatment of animals infested with the parasites bearing in mind their potential in the transmission of zoonotic diseases between humans and animals.
Anaplasmosis constituted 4.2% of the total cases that were reported. High seroprevalence of anaplasma antibodies have been detected in the wildlife and livestock´s in Kenya demonstrating that wildlife could serve as reservoirs of infection to domestic animals [34]. Human granulocytic anaplasmosis (HGA), a deer tick transmitted rickettsial infection caused by Anaplasma phagocytophilum, is a common cause of undifferentiated fever in the Northeast and Upper Midwest United States. Patients are often initially diagnosed with a mild viral infection, and illness readily resolves in most cases. However, as many as 3% may develop life threatening complications and nearly 1% die from the infection [35]. Two cases of human anaplasmosis through the process of blood transfusion was reported by [36]. Cases of babesiosis were reported in Taraba State for a period of five years with an average percentage of 1.9%. Bovine babesiosis is a tick-borne parasitic disease with significant morbidity and mortality in cattle. The economic losses can be considerable, especially when animals with no immunity are moved into an endemic area. Three species of Babesia cause most clinical cases in cattle [37]; Babesia bovis and B. bigemina are widespread in tropical and subtropical regions, while B. divergens circulates in parts of Europe and possibly in North Africa. The major vectors for B. bigemina and B. bovis are Rhipicephalus microplus and R. annulatus in some areas while Ixodes ricinus is the major vector for B. divergens [37]. Cattle that have recovered from acute babesiosis can remain asymptomatically infected, and recurrence of parasitemia can occur at irregular intervals. Humans are thought to become infected with B. divergens in tick bites [37]. The overall mortality rate for bovine babesiosis is reported to be 5-10%, even with treatment. Mortality can reach 50-100% in untreated animals infected with this organism [37]. The prognosis is guarded once hemoglobinuria develops, and CNS signs suggest a poor prognosis. In humans, infection by B. divergens has a case fatality rate of approximately 40% [37]. Dermatophilosis a disease of zoonotic importance was also indicated among the clinical cases in this study. Several studies have demonstrated the presence of dermatophilosis caused by dermatophilus congolensis in ruminants in Nigeria [38,39]. The disease can spread to the human populace through contact with infected animals.
Result obtained from our analysis indicated that among the diseases that affect poultry, Salmonellosis (enteric) in poultry is endemic in Taraba state. This indicates that Salmonellosis can be a source of infection to the public in the state based on documented evidence from numerous large outbreak investigations which demonstrated the transmission of human salmonellosis through contact with live chicks and ducklings [21,40,41]. Studies conducted on commercial chicken layer farms in Nigeria [30] on Salmonella serovars and their distribution indicated a farm prevalence of 43.6%. Salmonella specie may be present in about 65% of a flock with variable serotypes colonizing the gastrointestinal tract of poultry depending on the geographic location and time of the year for example S. enteritidis and S. typhimurium [42-44]. Many strains isolated from poultry that are responsible for food poisoning in humans´ demonstrated resistance to selected antibiotics. For this reason, much attention is drawn to eggs and poultry meat as sources of Salmonella [43]. Salmonellosis infection in birds is the primary source of infection in production environment [44,45] and in environmental contamination of poultry farms which plays a role in the transmission of Salmonella from live poultry to people since birds may shed Salmonella for long periods [46]. Egg content may be infected at the stage of forming due to bacterial colonization of the hen´s genital system leading to the production of infected eggs. Also, there may be environmental contamination of eggs with salmonella pathogen and possible penetration of the egg shell [47]. In humans, Salmonella specie was estimated to cause over 90 million diarrhea-associated diseases worldwide yearly with 85% of those cases associated with food poisoning [48] Also, other reports estimated the annual number of cases of salmonellosis globally ranging from 200 million to over 1 billion [47,49] with an expected world fatality rate associated with salmonellosis is over 150 thousand [50]. Human salmonellosis from contact with live poultry is a challenging, yet largely preventable, public health problem [46]. Prevention requires an integrated One Health approach including public and animal health officials collaborating with hatchery industry, feed store industry, healthcare providers, veterinarians, and backyard flock owners [46].
Some of the diseases of economic and public health importance reported in this study were CBPP, PPR, FMD and Newcastle which are transboundary in nature and are trade limiting [51]. Financial estimates of the burden of PPR, CBPP, trypanosomosis, NCD and ASF (TADs) in Nigeria amounted to NGN29.2 billion (182.3 million USD) [52]. This cost of inaction against the diseases were NGN 8.9 billion due to NCD in local chicken, PPR in sheep and goats (NGN 6.9 billion) and CBPP (NGN 2.2 billion) while the highest direct cost of inaction amounting to NGN 8.9 billion is due to NCD. All the TADs recorded in this study are vaccine preventable which indicates the low level of sensitization of farmers in disease prevention resulting in the use of antibiotics in the treatment of these food animals and they end up as meat on our tables. Food-producing animals are linked to humans via the food chain and the shared environment thus a one health approach to understanding how to control the AMR Situation Analysis and Recommendations threat becomes apparent [53,54]. The misuse of antimicrobials in the treatment of these diseases possess danger to the public health. The presence of veterinary drug residues in food products are a global health problem [51]. which needs to be curtailed for the safety and security food in order to protect the health of the public. Drugs are the most frequently detected chemical residues in food of animal origin, and majority of these are antimicrobials commonly used in veterinary practice in Nigeria [55]. The occurrence of contamination of edible animal products by antimicrobial and pesticide residues beyond the FAO/WHO permissible level is high in developing countries [51]. Lack of appropriate legislation to controlling the quality and distribution of veterinary pharmaceuticals and phytosanitary products in Nigeria market, will aggravate the health risk posed by drug residues if liberalization of the veterinary profession is allowed [51]. The nature, quantity and timing of antimicrobial administration determines the possible existence of antimicrobial residues in meats at the time of sale or consumption and potentially allows resistance selection from and through the food chain [51]. Analysis from 48 studies that tested resistance of a wide range of isolates using 55 antimicrobials belonging to different classes and generations showed resistant organisms were recovered from livestock (cattle, goats, pigs, sheep, camels and chickens) and from foods (dairy, vegetables and meats).
The presence of zoonoses and TADs among clinical cases in Taraba state is evident. Trends of these diseases have indicated that they are endemic in the state. Food borne zoonoses (helminthoses and Salmonellosis), vector borne zoonoses (Trypanosomosis, Babesiosis, Anaplasmosis), Dermatophilosis and ectoparasites in the state are treats to the health of the public. The high proportion of ectoparasites in the state points to the presence of anaplasmosis and babesiosis. In general, the welfare of the food animals in Taraba State needs to be taken care of in terms of treatment, prevention and control of these diseases so as to protect the health of the public. Development of antimicrobial resistance as a result of antibiotic abuse in veterinary services is a possible factor that cannot be ignored because of the extensive use of antibiotics in the treatment of TADs. This can further translate to treat to the public health through the food chain mechanism. Taraba state is strategically situated in Nigeria, with high population of livestock, sharing an international border with the republic of Cameroon and six states in the federation. This makes the influx of animals in and out of the state very easy due to the porosity of the inter and intra-national borders in Nigeria. The laxity in implementing the animal movement control act across the Control Posts nationwide is likely to play a great role in the transmission of zoonoses in the animals in the state with possible high risk of transmission to the public if the problem is not contained. The present information provided can aid the state in the promotion of disease-control policies, encourage on-farm good agricultural practices through proper training of farmers and adequate hygiene and sanitation in abattoirs and meat-processing plants, with the aim of protecting public health.
What is known about this topic
- Zoonotic diseases are major challenge to the public health sector globally;
- Majority of animal diseases are zoonotic in nature;
- Misuse of antimicrobials in animals has adverse effect on the health of the public.
What this study adds
- Majority of the animal diseases documented in this study were zoonotic, increasing the risk of transmission to humans;
- The extensive system of animal husbandry coupled with High proportion of Transboundary Animal Diseases (TADs) portends economic and public health challenges to Taraba State, destination States and countries;
- The indiscriminate use of antibiotic in the treatment of these animal infections by farmers contribute significantly to the global burden of antimicrobial resistance.
The authors declare no competing interests.
Emmanuel Yarai collected and collated data from the Taraba State Ministry of Agriculture. Ayi Vandi Kwaghe and Daniel Egom Okomah performed data analysis. Ayi Vandi Kwaghe drafted the manuscript. Mabel Kamweli Aworh, Emmanuel Awosanya, Chukwuma Umeokonkwo, Celestine Ameh and Junaid Kabir edited the manuscript. Chukwuma Umeokonkwo created the map of Nigeria. All authors read and accepted the final draft of these manuscript.
We acknowledge the Director Veterinary Services, State Ministry of Agriculture and Natural Resources from where these data were obtained from.
Table 1: sex and species distribution of diseases of ruminants and pigs in Taraba State, Nigeria, 2013 to 2017 (N=1,535,267)
Table 2: diseases of ruminants and pigs obtained from veterinary clinics/hospitals in Taraba State, Nigeria, 2013 to 2017 (N=1,535,267)
Table 3: diseases of poultry treated in Taraba State, Nigeria, 2013 to 2017 (N=237,671)
Figure 1: map of Taraba state (green colour) showing border states
Figure 2: total number of clinical cases reported per year from 2014 to 2017 in Taraba State
- Chlebicz A, Slizewska K. Campylobacteriosis, Salmonellosis, Yersiniosis, and Listeriosis as Zoonotic Foodborne Diseases: a review. Int J Environ Res Public Health. 2018 Apr 26;15(5):863. PubMed | Google Scholar
- Okello A, Gibbs E, Vandersmissen A, Welburn S. One Health and the neglected zoonoses: turning rhetoric into reality. Vet Rec. 2011;169(11):281-5. PubMed | Google Scholar
- Lorusso V, Picozzi K, de Bronsvoort BM, Majekodunmi A, Dongkum C, Balak G et al. Ixodid ticks of traditionally managed cattle in central Nigeria: where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasit Vectors. 2013 Jun 7;6:171. PubMed | Google Scholar
- Food Safety Authority E, Boelaert F, Van der Stede Y, Nagy K, Rizzi V, Garcia Fierro R et al. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2016 Acknowledgements: EFSA and the ECDC wish to thank the members of the Scientific Network for Zoonoses Monitoring Data and the Food and Waterborne Diseases and Zoonoses Network, who provided the data and reviewed the report; the members of the Scientific Network for Zoonoses Monitoring Data for their endorsement of this scientific report; the EFSA staff members. EFSA J. 2017;15(12):5077.
- Food and Agriculture Organization. Livestock and livelihoods spotlight NIGERIA: Cattle and Poutry Sectors. FAO. 2018.
- Oruonye ED. An Assessment of the Trends of Climatic Variables in Taraba State Nigeria. Type Double Blind Peer Rev Int Res J Publ Glob Journals Inc. 2014;14. Google Scholar
- Oruonye ED, Abbas B. The Geography of Taraba State Nigeria: Natures Gift to the Nation - AbeBooks - Emeka Daniel Oruonye; Abbas Bashir: 3846504513. LAP Publishing Company, Germany; 2011.
- Andrews SJ. The life cycle of Fasciola hepatica. In Fasciolosis, JP Dalton, editor Oxford, UK CABI. 1999;1-544.
- Mas-Coma S, Bargues MD, Valero MA. Fascioliasis and other plant-borne trematode zoonoses. Int J Parasitol. 2005 Oct 1;35(11-12):1255-78. PubMed | Google Scholar
- Mas-Coma S, Anglés R, Esteban JG, Bargues MD, Buchon P, Franken M et al. The Northern Bolivian Altiplano: a region highly endemic for human fascioliasis. Trop Med Int Heal. 1999 Jun 6;4(6):454-67. PubMed | Google Scholar
- Esteban J-G, Gonzalez C, Curtale F, Muñoz-Antoli C, Valero MA, Bargues MD et al. Hyperendemic fascioliasis associated with schistosomiasis in villages in the Nile Delta of Egypt. Am J Trop Med Hyg. 2003 Oct;69(4):429-37. PubMed | Google Scholar
- Ekong PS, Juryit R, Dika NM, Nguku P, Musenero M. Prevalence and risk factors for zoonotic helminth infection among humans and animals - Jos, Nigeria, 2005-2009. Pan Afr Med J. 2012;12:6. PubMed | Google Scholar
- Karshima SN, Maikai B-V, Kwada J. Helminths of veterinary and zoonotic importance in Nigerian ruminants: a 46-year meta-analysis (1970-2016) of their prevalence and distribution. Infect Dis Poverty. 2018;7(1):52. PubMed | Google Scholar
- Boa ME, Mahundi EA, Kassuku AA, Willingham AL, Kyvsgaard NC. Epidemiological survey of swine cysticercosis using ante-mortem and post-mortem examination tests in the southern highlands of Tanzania. Vet Parasitol. 2006 Jun 30;139(1-3):249-55. PubMed | Google Scholar
- Prakash A, Kumar GS, Rout M, Nagarajan K, Kumar R. Neurocysticercosis in free roaming pigs - a slaughterhouse survey. Trop Anim Health Prod. 2007 Aug 27;39(6):391-4. PubMed | Google Scholar
- Pawlowski Z, Allan J, Sarti E. Control of Taenia solium taeniasis/cysticercosis: From research towards implementation. Int J Parasitol. 2005 Oct;35(11-12):1221-32. PubMed | Google Scholar
- Lateef M, Zargar SA, Khan AR, Nazir M, Shoukat A. Successful treatment of niclosamide- and praziquantel-resistant beef tapeworm infection with nitazoxanide. Int J Infect Dis. 2008 Jan 1;12(1):80-2. PubMed | Google Scholar
- Allan JC, Craig PS. Coproantigens in taeniasis and echinococcosis. Parasitol Int. 2006 Jan;55:S75-80. PubMed | Google Scholar
- Garcia HH, Moro PL, Schantz PM. Zoonotic helminth infections of humans: echinococcosis, cysticercosis and fascioliasis. Curr Opin Infect Dis. 2007 Oct;20(5):489-94. PubMed | Google Scholar
- Jauro S, Musa Z, Onyilokwu SA, Yakubu C, Musa JA. Occurrence of Salmonella in ruminants and camel meat in Maiduguri, Nigeria and their antibiotic resistant pattern. J Adv Vet Anim Res. 2017;4(3):227-33. Google Scholar
- Hedican E, Miller B, Ziemer B, LeMaster P, Jawahir S, Leano F et al. Salmonellosis Outbreak Due to Chicken Contact Leading to a Foodborne Outbreak Associated with Infected Delicatessen Workers. Foodborne Pathog Dis. 2010 Aug;7(8):995-7. PubMed | Google Scholar
- Kassaye BK, Hassen DJ, Leja KA, Tsegaye B. Study on Prevalence and Distribution of Salmonella Isolates from Apparently Healthy Sheep and Goats Slaughtered at Addis Ababa Abattoir Enterprise,Ethiopia. J Vet Sci Technol. 2015 Oct 16;06(06):1-5. Google Scholar
- Anene BM, Chime AB, Jibike GI, Anika SM. Prevalence of trypanosomiasis in Zebu cattle at Obudu ranch-a tsetse-free zone in Nigeria. Prev Vet Med. 1991 May;10(4):257-60. Google Scholar
- Odeniran PO, Ademola IO. A meta-analysis of the prevalence of African animal trypanosomiasis in Nigeria from 1960 to 2017. Parasit Vectors. 2018 Dec 2;11(1):280. PubMed | Google Scholar
- Majekodunmi AO, Fajinmi A, Dongkum C, Picozzi K, Thrusfield M V, Welburn SC. A longitudinal survey of African animal trypanosomiasis in domestic cattle on the Jos Plateau, Nigeria: prevalence, distribution and risk factors. Parasites & vectors. 2013. Google Scholar
- Oguntomole O, Nwaeze U, Eremeeva ME. Tick-, Flea-, and Louse-Borne Diseases of Public Health and Veterinary Significance in Nigeria. Trop Med Infect Dis. 2018 Jan 3;3(1). PubMed | Google Scholar
- Ogo NI, de Mera IGF, Galindo RC, Okubanjo OO, Inuwa HM, Agbede RIS et al. Molecular identification of tick-borne pathogens in Nigerian ticks. Vet Parasitol. 2012 Jul 6;187(3-4):572-7. PubMed | Google Scholar
- Reye AL, Arinola OG, Hübschen JM, Muller CP. Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl Environ Microbiol. 2012 Apr;78(8):2562-8. PubMed | Google Scholar
- Paul BT, Bello AM, Ngari O, Mana HP, Gadzama MA, Abba A et al. Risk factors of haemoparasites and some haematological parameters of slaughtered trade cattle in Maiduguri, Nigeria. J Vet Med Anim Heal. 2016 Aug 31;8(8):83-8. Google Scholar
- Fagbamila IO, Barco L, Mancin M, Kwaga J, Ngulukun SS, Zavagnin P et al. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017 Mar 9;12(3):e0173097. PubMed | Google Scholar
- Opara N, Santali A, Mohammed C. Prevalence of Haemoparasites of Small Ruminants in Lafia Nassarawa State: A Guinea Savannah Zone of Nigeria. J Vet Adv. 2016;6(6):1251. Google Scholar
- Lorusso V, Wijnveld M, Majekodunmi AO, Dongkum C, Fajinmi A, Dogo AG et al. Tick-borne pathogens of zoonotic and veterinary importance in Nigerian cattle. Parasites & vectors. 2016. Google Scholar
- Rony SA, Mondal MMH, Begum N, Islam MA, Affroze S. Epidemiology of ectoparasitic infestations in cattle at bhawal forest area, gazipur. Bangladesh Journal of Veterinary Medicine. 2010. Google Scholar
- Ngeranwa JJN, Shompole SP, Venter EH, Wambugu A, Crafford JE, Penzhorn BL. Detection of Anaplasma antibodies in wildlife and domestic species in wildlife-livestock interface areas of Kenya by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. Onderstepoort J Vet Res. 2008 Sep;75(3):199-205. PubMed | Google Scholar
- Bakken JS, Dumler JS. Human granulocytic anaplasmosis. Infect Dis Clin North Am. 2015 Jun;29(2):341-55. PubMed | Google Scholar
- Annen K, Friedman K, Eshoa C, Horowitz M, Gottschall J, Straus T. Two Cases of Transfusion-Transmitted Anaplasma phagocytophilum. Am J Clin Pathol. 2012 Apr 1;137(4):562-5. PubMed | Google Scholar
- Spickler AR. Bovine Babesiosis. College of Veterinary Medicine. 2018.
- Dalis JS, Kazeem HM, Makinde AA, Fatihu MY. Distribution of Lesions of Dermatophilosis in Cattle Sheep and Goats in Zaria and Jos, Nigeria. J Anim Vet Adv. 2009;8(2):385-8. Google Scholar
- Shaibu SJ, Kazeem HM, Abdullahi US, Fatihu MY, Yakubu B, Makinde AA et al. Direct detection of Dermatophilus congolensis from skin scabs of dermatophilosis infected animals by polymerase chain reaction. J Food, Agric Environ. 2010;8(3&4):577-9. Google Scholar
- Loharikar A, Briere E, Schwensohn C, Weninger S, Wagendorf J, Scheftel J et al. Four Multistate Outbreaks of Human Salmonella Infections Associated with Live Poultry Contact, United States, 2009. Zoonoses Public Health. 2012 Aug;59(5):347-54. PubMed | Google Scholar
- Centers for Disease Control and Prevention (CDC). Notes from the field: Multistate outbreak of human Salmonella typhimurium infections linked to contact with pet hedgehogs - United States, 2011-2013. MMWR Morb Mortal Wkly Rep. 2013 Feb 1;62(4):73.
- Barua H, Biswas PK, Olsen KEP, Shil SK, Christensen JP. Molecular Characterization of Motile Serovars of Salmonella enterica from Breeder and Commercial Broiler Poultry Farms in Bangladesh. Chakravortty D, editor PLoS One. 2013 Mar 6;8(3):e57811. PubMed | Google Scholar
- Saravanan S, Purushothaman V, Murthy TRGK, Sukumar K, Srinivasan P, Gowthaman V et al. Molecular Epidemiology of Nontyphoidal Salmonella in Poultry and Poultry Products in India: Implications for Human Health. Indian J Microbiol. 2015 Sep;55(3):319-26. PubMed | Google Scholar
- Nidaullah H, Abirami N, Kamal Shamila-Syuhada A, Chuah L-O, Nurul H, Tan TP et al. Prevalence of Salmonella in poultry processing environments in wet markets in Penang and Perlis, Malaysia. 2017;10(3): 286-92 PubMed |
- Kallapura G, Morgan MJ, Pumford NR, Bielke LR, Wolfenden AD, Faulkner OB et al. Evaluation of the respiratory route as a viable portal of entry for Salmonella in poultry via intratracheal challenge of Salmonella Enteritidis and Salmonella Typhimurium. Poult Sci. 2014 Feb;93(2):340-6.. PubMed | Google Scholar
- Behravesh CB, Brinson D, Hopkins BA, Gomez TM. Backyard Poultry Flocks and Salmonellosis: A Recurring, Yet Preventable Public Health Challenge. Clin Infect Dis. 2014 May 15;58(10):1432-8. PubMed | Google Scholar
- Whiley H, Ross K. Salmonella and Eggs: From Production to Plate. Int J Environ Res Public Health. 2015 Feb 26;12(3):2543-56. PubMed | Google Scholar
- Hung Y-T, Lay C-J, Wang C-L, Koo M. Characteristics of nontyphoidal Salmonella gastroenteritis in Taiwanese children: A 9-year period retrospective medical record review. J Infect Public Health. 2017 Sep 1;10(5):518-21. PubMed | Google Scholar
- Bierschenk D, Boucher D, Schroder K. Salmonella-induced inflammasome activation in humans. Mol Immunol. 2017;86:38-43. PubMed | Google Scholar
- de Jong HK, Parry CM, van der Poll T, Wiersinga WJ. Host-Pathogen Interaction in Invasive Salmonellosis. Chitnis CE, editor PLoS Pathog. 2012 Oct 4;8(10):e1002933. PubMed | Google Scholar
- Federal Ministries of Agriculture E and H . Antimicrobial Us and Resistance in Nigeria: Situation Analysis and Recommendations. (FMAEH). 2017.
- Fadiga ML, Jost C, Ihedioha J. Financial costs of disease burden, morbidity and mortality from priority livestock diseases in Nigeria: Disease burden and cost-benefit analysis of targeted interventions. 2013.
- Karczmarczyk M, Martins M, Quinn T, Leonard N, Fanning S. Mechanisms of fluoroquinolone resistance in Escherichia coli isolates from food-producing animals. Appl Environ Microbiol. 2011 Oct;77(20):7113-20. PubMed | Google Scholar
- Pamley J, Leung Z, Léger D, Finley R, Irwin R, Pintar K et al. One Health and Food Saftey-The Canadian Experience: A Holistic Approach Toward Enteric Bacterial Pathogens and Antimicrobial Resistance Surveillance. Improving Food Safety Through a One Health Approach. National Academies Press. 2012.
- Aliu Y. Veterinary drug residues in Nigeria´s food. national awareness training programme on food contaminants and residues Women development centre, Kaduna Nigeria. 2004.