Prevalence of Dengue virus among healthy blood donors in Mombasa County, Kenya
Festus Mulakoli, George Gachara, Eric Ndombi, Samoel Khamadi
Corresponding author: Festus Mulakoli, School of Nursing and Midwifery, Aga Khan University/Department of Medical Laboratory Sciences, Kenyatta University, Nairobi, Kenya 
Received: 06 Jan 2024 - Accepted: 03 Feb 2024 - Published: 29 Feb 2024
Domain: Epidemiology,Immunology,Virology
Keywords: Seroprevalence, epidemics, outbreak, transfusion-transmitted Dengue, Kenya
©Festus Mulakoli et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Festus Mulakoli et al. Prevalence of Dengue virus among healthy blood donors in Mombasa County, Kenya. PAMJ-One Health. 2024;13:6. [doi: 10.11604/pamj-oh.2024.13.6.42602]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/13/6/full
Prevalence of Dengue virus among healthy blood donors in Mombasa County, Kenya
![]() Festus Mulakoli1,2,&,
Festus Mulakoli1,2,&, ![]() George Gachara2, Eric Ndombi3,
George Gachara2, Eric Ndombi3, ![]() Samoel Khamadi4
Samoel Khamadi4
&Corresponding author
Introduction: Dengue fever (DF) is a viral infection caused by a flavivirus called Dengue virus. The virus has four known serotypes (named DENV 1-4) that circulate between humans and Aedes mosquitoes throughout the tropical region of the world. The virus is transmitted primarily by the bite of an infected Aedes aegypti or, to a lesser extent, Aedes albopictus. Current evidence from published case studies shows that blood transfusions can transmit Dengue infection in hyperendemic regions in the tropics. It is important to note that 75% of people infected with DENV show no symptoms. Therefore, an infected individual could be accepted as a blood donor and spread the disease. In Kenya, frequent Dengue outbreaks have been reported in the coastal counties of Mombasa, Kilifi, and Kwale in recent years. This study aimed to determine the seroprevalence of Dengue virus among blood donors in a selected endemic region of the Republic of Kenya.
Methods: the researchers used a cross-sectional research design to collect data from blood donors in two selected counties in Kenya in 2023. A self-directed questionnaire was used to collect sociodemographic data and risk factors associated with Dengue fever from consenting participants. Additionally, a 5-ml sample of blood was collected and serologically analyzed for anti-Dengue IgG, IgM, and NS1 using a commercial rapid Dengue testing duo kit (Bioline™ DENGUE DUO (Dengue NS1 Ag + IgG/IgM)). The data were summarized and presented using tables and bar graphs.
Results: at the end of the study, the researchers recruited 103 participants from the selected study sites in Mombasa County. Most of the research participants were men between 20 and 30 years of age. The prevalence of Dengue virus seromarkers was 24%, 11%, and 2% for IgG, IgM, and NS1, respectively. These were detected among young adult donors between the ages of 20 and 30 years. Statistically, there was an association between anti-Dengue IgM positivity with a history of admission (p-value = 0.0015), fever in the last 6 months (p-value = 0.0015) and a history of living with a DF infected person in the last 6 months (p-value = 0.011). Similarly, there was a statistically significant association between anti-Dengue IgG positivity and length of stay in Mombasa County (p-value = 0.005), history of admission in the last 6 months (p-value =0.003), history of fever in the last 6 months (p-value = 0.004) and lived with a victim of Dengue fever in the last 6 months (p-value = 0.02) in a 95% confidence interval.
Conclusion: according to the study findings, a sizeable proportion of eligible blood donors in Mombasa are Dengue virus-infected, some possibly carrying the virus without showing symptoms. The study identified IgG and IgM as the most prevalent serological markers. To protect blood recipients in Mombasa County and other Dengue-endemic counties such as Kilifi, Kwale, Lamu, and Taita Taveta, it is recommended that blood donors in these regions undergo regular screening, particularly during Dengue outbreaks.
Dengue fever is a viral infection that has recently been discovered to be transmissible through blood transfusions. Although Aedes mosquitoes are the primary vector of Dengue, they can also be transmitted via blood transfusions and organ transplants. This means that if a person receives blood from an infected donor, they can contract the virus through blood transfusions [1-3]. Various studies conducted in hyperendemic countries have reported five cases of Dengue hemorrhagic fever because of transfusion-transmitted Dengue [4-6]. The Dengue virus, a mosquito-borne disease, is endemic in tropical and subtropical areas of the world. Dengue fever has spread to 128 countries in the tropical and subtropical regions of the world. According to estimates, half of the world's population is at risk of contracting the Dengue virus. The symptoms of Dengue fever can range from mild to severe and include high fever, headache, muscle and joint pain, nausea, vomiting, and rash. In severe cases, Dengue fever can lead to Dengue hemorrhagic fever, which can be life-threatening. Each year, approximately 25,000 people die from the Dengue virus [7,8]. There is evidence that transfusions can spread Dengue infection in areas where the virus is persistent. Emerging infectious diseases are a problem for blood transfusion services right now. As a result, developed countries are required to perform additional tests that would be prohibitively expensive in developing countries such as Kenya [9]. Babesia, Chagas disease, chikungunya, zika virus, Dengue virus (DENV), hepatitis E virus, West Nile virus, human T lymphocyte virus, and Ross River virus are examples of emerging diseases in blood transfusions [10].
Studies conducted in countries such as Brazil, India, China, Saudi Arabia, Ghana, Tanzania, and Cameroon have concluded that the Dengue virus poses a threat to safety and availability in their respective countries [11-13]. The Dengue virus can now be spread through blood and tissue transplants, besides the traditional mosquito bite method. Several studies have shown that Dengue can be transmitted by blood transfusion. However, the first case was reported in China in 2002 and in Singapore in 2008 by Tambyah et al. [14], Chuang et al. [15]. According to their findings, patients who received blood transfusions from asymptomatic blood donors quickly developed Dengue-like symptoms. Comparable studies in Brazil, Puerto Rico, and Singapore during the 2016 outbreaks reported a 0.5% incidence rate of Dengue viremia among asymptomatic blood donors [6,16]. Ashshi [17] in their study among healthy blood donors in Saudi Arabia reported a seroprevalence between 1 and 7% for NS1, IgM, and IgG antibodies. Similarly, a study by Rodriguez Rodriguez et al. [18] using the enzyme-linked immunosorbent assay (ELISA) technique in blood donors in the northeastern region of Mexico discovered IgG and IgM levels of 59% and 2%, respectively. However, reverse transcription-polymerase chain reaction (RT-PCR) analysis did not find any evidence of viremia. These scenarios are most often observed in healed Dengue infections and are a technical limitation of the RT-PCR assay [19]. In India, the country most affected by the Dengue virus, a study by Kulkarni et al. [20] in the Pune region of western India reported seropositivity of 0.64 % and 6.4% for NS1 and IgM, respectively, in 2017.
Little is known about the prevalence of Dengue virus among blood donors in Africa, and the probability of Dengue fever caused by transfusion-transmitted Dengue (TTD) is high [21]. Studies conducted in some countries have detected Dengue markers in asymptomatic blood donors. For example, a study by Tchuandom et al. [22] among Cameroonian blood donors reported a prevalence rate of 5% for all serological markers using a simple immunochromatographic diagnostic kit. In Tanzania, a similar study by Vairo et al. [23] reported a prevalence of 50.6% anti-Dengue (IgG) among blood donors in Zanzibar. Unfortunately, there is less research output from African countries compared to other endemic regions of the world. To fill these gaps, a lot of effort and commitment are required to conduct research studies on blood safety in Africa [24].
The current problem is exacerbated in African countries because of limited resources, a high incidence of known transfusion-transmitted infection (TTI) a lack of blood supplies, and a shortage of blood transfusion specialists. As a result, the impact of these new infectious diseases on blood safety and the associated costs adds to the already significant financial burden on blood transfusion services in African countries [25]. Dengue fever is endemic in Africa in several countries, including Kenya, Tanzania, Sudan, Egypt, Nigeria, Cameroon, Burkina Faso, and Senegal. These countries have previously experienced outbreaks of Dengue fever, and the disease continues to be a major public health concern.
Prevention of Dengue fever involves taking measures to avoid mosquito bites, such as using insect repellent, wearing long-sleeve clothing, and staying in air-conditioned or well-screened areas. Currently, there is no specific treatment for Dengue fever, but supportive care can help manage symptoms and prevent complications. To prevent Dengue fever transmitted by transfusions, some blood transfusion services in developed countries have added Dengue virus screens to their testing algorithm as a precautionary measure. This precautionary approach is intended to ensure the safety of the blood supply and the continuous availability of blood during outbreaks of Dengue fever [26,27].
The virus is prevalent in Kwale, Kilifi, Mandera, Lamu, Taita-Taveta, and Mombasa counties in Kenya. Dengue virus outbreaks have occurred since 2004, the most recent occurring in 2017. According to an epidemiological study conducted on febrile patients in Mombasa County using IgM as a marker, the prevalence of Dengue virus was 41% among febrile patients. According to the findings, DENV-1 was the most common serotype in samples collected between 2011 and 2014, followed by DENV-2 (38.5%) and DENV-3 (17.4%) [28]. Other major outbreaks have been observed in Kilifi, Lamu, Kwale, and Mandera between 2011 and 2018 [29,30]. Limited information is available on the impact of disease outbreaks on the safety of blood supplies in affected regions. This study addressed two research questions: (i) What is the prevalence of Dengue virus among blood donors in Mombasa County; and (ii) What are the demographic characteristics and risk factors associated with Dengue virus positivity among blood donors? Therefore we aimed to investigate the prevalence of the Dengue virus among healthy blood donors in Mombasa County in Kenya and to analyze its potential implications for the safety of blood transfusions.
Study design and setting: the researchers used a cross-sectional study design guided by the STROBE guidelines [31] to collect data at a private blood donation center in Mombasa County. The donation center is located within a leading private tertiary healthcare institution in the county. The researchers collected their data between December 2022 and July 2023 using approved data collection tools. Validated questionnaires were used to collect study-related information from blood donors at the study site. Additionally, blood samples were collected from consenting donors after meeting the minimum qualification of a blood donor according to institutional guidelines. Convenient sampling was used to sample eligible blood donors for this study. The principal investigator (PI) of the study provided a self-administered questionnaire to all consenting blood donors during the field visit. The dependent variable for the study included the donor's residence, the duration of stay at the study site, travel history to countries in the Middle East, use of insect repellants, environmental conditions in his home space, sex, age, religion, medical history, and level of education. The independent variable for this study was seropositivity for Dengue IgG, IgM, and NS1 antigen. These samples received unique identifiers to conceal the identity of the research participants. This was followed by the test of three Dengue viral markers (IgG, IgM, and NS1 antigen) using a commercial rapid Dengue testing duo kit (Bioline™ DENGUE DUO (DENGUE NS1 Ag + IgG/IgM)).
Study population and sampling procedure: the target population for the study was individuals who presented themselves as voluntary blood donors during the study period. The study included blood donors who were between 16 and 65 years of age and gave their consent to participate. The study excluded people who had not lived in the study area for the last six months and those who had a fever in the last two weeks.
Sample size determination: the researchers used an online sample size calculator [32] where a prevalence of NS1 of 6.1% was used as reported by Tchuandom et al. [22], with a confidence level of 95% and a margin of error of 5. The minimum sample size for this study was 89 blood donors after the calculation.
Study variables and data sources/measurement: the dependent variables of interest for this study were anti-Dengue IgG, IgM, and NS1 antigen. The definition of Dengue fever was characterized by positivity for IgM or NS1. On the other hand, the independent variables were sociodemographic factors (age, sex, religion, level of education) and risk determinants (use of insect repellents, outdoor activities, history of travel to Dengue-endemic regions, stagnant water, and congested human dwellings) for the transmission of the Dengue virus. A self-administered questionnaire (English and Kiswahili) was used to capture relevant information (donor demographics and serology results). Aseptically collected blood samples were analyzed for the presence of the Dengue seromarkers IgM, IgG, and NS1. A commercial immunochromatography kit was used for this purpose.
Laboratory testing: at the study site, serum samples were serologically tested for NS1, IgM, and IgG using a combined Rapid test cassette (Bioline™ DENGUE DUO, Abbott Park, Illinois, United States) following the manufacturer's instructions. The results obtained were entered into the individual donor questionnaire and recorded as positive or negative for the markers.
Data management: the data collected were entered into an Excel spreadsheet, cleaned, and then entered into SPSS version 20. A Chi-square test (χ2) at a 95 confidence interval was used to examine the relationships between binary or nominal variables. The Chi-square test was used to assess the characteristics of the relationship between the predictor variables and the outcome variables. The predictor variables were significant if their p-value was less than 0.05 at a 95% confidence interval.
Ethical considerations: the Aga Khan University Institutional Scientific Ethics Research Committee granted ethical approval with reference number 2022/ISERC-10(v3) (AKU-ISERC). Before data collection, a research permit with reference number 239512 was obtained from the National Commission for Science, Technology, and Innovation (NACOSTI).
This study recruited 103 blood donors after meeting the minimum requirements for an eligible donor as described in the donor questionnaire. Most of them n-74 (71.8%) were between 21 and 30 years old. This was followed by people between 31 and 40 years of age at n=23 (22.3%). Males were the main blood donors at n= 87 (84.5%) who volunteered to donate blood during the study period. Lastly, n=74 (71.8%) of the blood donors indicated that they were Christians (Table 1).
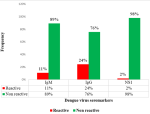
Prevalence of Dengue virus among selected research participants: the prevalence of Dengue virus was 11%, 24%, and 2 % for IgM, IgG, and NS1, respectively. The most dominant marker for Dengue fever was anti-Dengue IgG, followed by anti-Dengue IgM. Only a few donors had detectable NS1 antigens in their blood (Figure 1). During the study period, these markers were distributed differently between various age groups of blood donors. Blood donors between the ages of 21-30 and 41-50 years had a higher prevalence of anti-Dengue IgM and IgG (Figure 2).
Sociodemographic characteristics and risk factors are associated with Dengue virus positivity: to determine the association between independent variables (age, gender, education, use of insect repellant use, etc.) and dependent variable (Dengue seropositivity), we performed a Chi-square analysis (χ2). The analysis showed that there was a significant association between anti-Dengue IgM positivity with a history of admission (p=0.0015), having had a fever in the last six months (p=0.0015), and a history of living with a Dengue fever-infected person in the last six months (p=0.011) at 95 CI (Table 2). However, there was no correlation between the dependent variable NS1 positivity and the independent variables of interest in this study (Table 3). Similarly, there was a statistically significant association between anti-Dengue IgG positivity and length of stay in Mombasa County (p=0.005), history of admission in the last six months (p=0.003), history of fever in the last six months (p = 0.004) and lived with a Dengue fever-infected person in the last six months (p= 0.02) (Table 4).
The prevalence of Dengue virus infection among unpaid voluntary donors in an endemic region around the world is a matter of great concern for the safety and availability of blood products. The rapid spread of the virus is the subject of discussion among scholars involved in blood safety. In this study, our findings show that a good number of blood donors were exposed to the Dengue virus at the time of data collection. This confirms that Mombasa County is an endemic region of the Dengue virus in the Republic of Kenya [33]. This finding is similar to the 13% that was reported in the 2013 household survey by Ellis et al. [34]. Exposure to Dengue virus (IgG) was higher than that reported by Koech [35] among blood donors in Nairobi (11.1%), Kisumu (5.4%), and Eldoret (2.2%). This difference is because of the regional variation in the prevalence of different counties in Kenya.
Regionally, our findings were higher than the 2.2 % reported in a study by Muhibi et al. [13] among blood donors in Nigeria and 0.0% among Egyptian blood donors by Abd El-Wahab et al. [36]. Similarly, our findings were reported to be less than 24.8% among blood donors in Cameroon, where the prevalence of IgM, IgG, and NS1 was reported at 12.3%, 4.5%, and 6.1% [22]. Our exposure [37] to Dengue virus was also less than 50.6% reported in Zanzibar by Vairo et al. [23], and 43.6% in Ghana by Narkwa et al. [38]. Variation in exposure to Dengue virus could be attributed to the test method used in these studies that predispose to cross-reactivity with other flavivirus infections [39]. Therefore, it is important to exercise caution when interpreting the results of these studies before concluding on the implications of the Dengue virus on blood safety and availability.
Globally, our findings for anti-Dengue IgG were higher than 1.4% reported in a study in Brazil by Ribas-Silva [40], 4.21% in another study by Slavov et al. [41] the same country, 3.4% in donor blood samples in China [42], and 2.25% in the same country [43]. Similarly, our findings were comparable to 26.53% reported among blood donors in Yunnan province by Li et al. [44]. However, our findings were lower than the 17.9% reported in a study in Jordan by Swedan [45]. Our prevalence rate of 2% was less than NS-1 0.54% reported in India by Jain et al. [46]. A similar observation was observed in anti-Dengue IgM, where our findings were similar to 11.23%, but less than 6.74% reported in two studies among donors in India [47,48]. However, our findings were higher than the 0.78% reported in China by Gao et al. [49], and 5.5% by Ashshi et al. [27]. These findings suggest that, if the plasma sample tests were positive for IgM, it is likely that the infection occurred between one and two months before the sample was collected. This indicates that the virus is still actively circulating in the community and that more aggressive measures are needed to contain its spread. The presence of DENV-NS1 antigen and/or anti-DENV IgM antibody in the tested donors indicates that they are in the asymptomatic viremic infectious stage with DENV during donation time, while the high prevalence of anti-DENV IgG suggests that Dengue disease is highly prevalent in this region.
There was a variation in the prevalence of NS1 reported in our study compared to what is reported elsewhere. For example, our findings were higher than 0.56%, 0.9%, and 0.54%, as reported in three studies from India by Raj, Shashindran et al. [47] Jain, et al. [46], Remakanth et al. [48]. However, our findings were lower than the 5.3% reported in a study in Saudi Arabia by Ashshi et al. [27]. The presence of NS1, a protein associated with Dengue virus infection, in Mombasa blood donors, poses a serious threat to the safety of donated blood in this region. This indicates that individuals could have potentially transmitted the virus to recipients of these blood products. As Dengue infection can have severe consequences, including death, it is essential to implement strict screening procedures to prevent infected blood from entering the blood supply. The findings on the NS1 protein are consistent with those of a previous study, indicating the reliability of our research. This provides further evidence for the existing body of knowledge on NS1. The congruent results of both investigations suggest that NS1 plays a crucial role in the biological processes studied and can be considered a significant factor in the development of potential treatments or therapies [50-53].
Dengue fever is a pressing issue in numerous regions of the world and poses a grave threat to public health. Blood transfusion is a known mode of transmission that poses a significant hazard to blood recipients. Although the Aedes mosquito is the main vector of Dengue transmission, studies indicate that the virus can also be transmitted by blood transfusions. As such, people who donate or receive blood may be at risk of contracting the virus and subsequently developing Dengue fever [1,36,48,49].
Study limitations and bias: this study was conducted during a dry season in the study area and in a single blood donation center in the vast coastal region of the Republic of Kenya. Therefore, the prevalence obtained cannot be generalized to present the entire coastal region.
There is a high prevalence of Dengue virus among blood donors in Mombasa County, with a majority having previously been exposed to the Dengue virus. Therefore, screening of blood donors in this region for Dengue virus will be a good measure to protect blood recipients from Dengue fever.
What is known about this topic
- Mombasa County is a known endemic region of Dengue fever in the Republic of Kenya;
- Studies in other global regions have shown a varying prevalence of the Dengue virus among blood donors.
What this study adds
- Documented the first study of Dengue virus among blood donors in Mombasa County, Kenya;
- Illustrated the need to introduce Dengue virus screening among blood donors in all endemic counties of the Republic of Kenya and other African countries.
The authors declare no competing interests.
The study was partially funded by the Aga Khan University Deans Fund (USD 5850).
The initial concept of the study was developed by Festus Mulakoli. George Gachara and Eric Ndombi reviewed the concepts and made additional amendments to the final concept. Samoel Khamadi reviewed the method section of the manuscript. The four authors critically reviewed and co-authored the final manuscript before submission for publication.
We express our appreciation for the support we received from the staff, our participants, and our colleagues. The authors also thank the Dean of the Aga Khan University School of Nursing for financial support.
Table 1: sociodemographic data of blood donors for the study
Table 2: correlation between sociodemographic/risk factors and anti-Dengue IgM positivity
Table 3: correlation between sociodemographic/risk factors and NS1 positivity
Table 4: correlation between sociodemographic/risk factors and anti-Dengue IgG positivity
Figure 1: prevalence of Dengue seromarkers among blood donors in Mombasa County
Figure 2: distribution of Dengue seromarkers among different age groups of selected blood donors in Mombasa County
- Huang Y, Forshee RA, Fares-Gusmao R, Chancey C, Rios M, Anderson SA et al. A risk assessment model for transfusion transmission of dengue. Lett Appl Microbiol. 2022 Nov;75(5):1330-5. PubMed | Google Scholar
- Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT et al. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013 May;19(5):787-9. PubMed | Google Scholar
- He M, Wang J, Chen L, Liu J, Zeng P. The Impact of Emerging Infectious Diseases on Chinese Blood Safety. Transfusion Medicine Reviews. 2017;31(2):94-101. PubMed | Google Scholar
- Pozzetto B, Memmi M, Garraud O. Is transfusion-transmitted dengue fever a potential public health threat? World J Virol. 2015 May 12;4(2):113-2. PubMed | Google Scholar
- Yan G, Tambyah P. Transfusion-Transmitted Infections: Lessons From Dengue in Taiwan. J Infect Dis. 2022 May 4;225(9):1497-1499. PubMed | Google Scholar
- Matos D, Tomashek KM, Perez-Padilla J, Muñoz-Jordán J, Hunsperger E, Horiuchi K et al. Probable and possible transfusion-transmitted dengue associated with NS1 antigen-negative but RNA confirmed-positive red blood cells. Transfusion. 2016 Jan;56(1):215-22.. PubMed | Google Scholar
- Jing Q, Wang M. Dengue epidemiology. Global Health Journal. 2019 Jun 1;3(2):37-45. Google Scholar
- Yang X, Quam MBM, Zhang T, Sang S. Global burden for dengue and the evolving pattern in the past 30 years. J Travel Med. 2021 Dec 29;28(8):taab146. PubMed | Google Scholar
- Perera L, De Zoysa N, Jayarajah U, Senanayake N, De Zoysa I, Seneviratne SL. Transfusion-transmissible dengue infections. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2020;114(11):866-82. PubMed | Google Scholar
- Stramer SL. The potential threat to blood transfusion safety of emerging infectious disease agents. Clin Adv Hematol Oncol. 2015 Jul;13(7):420-2. PubMed | Google Scholar
- Basavarajegowda A, Remakanth R, Dhodapkar R. Prevalence of dengue NS1 antigenemia among healthy blood donors in a tertiary care hospital in Southern India. Asian J Transfus Sci. 2021 Jul-Dec;15(2):140-145. PubMed | Google Scholar
- Custer B, Goncalez T, Gao K, Brambilla D, Proietti AC, Mendrone A, Loureiro P, Lopes ME, Capuani L, Busch MP, Sabino E. Zika, Chikungunya and Dengue Virus Incident Infections in Blood Donors in Brazil in 2016: Implications for Blood Safety and Public Health Surveillance. In2017 AABB Annual Meeting 2017 Oct 7. AABB. Google Scholar
- Muhibi MA, Adeleke MA, Shittu BT, Jeremiah ZA. Dengue Virus Infection among Voluntary Blood Donors in Osogbo, Southwestern Nigeria. Am J Biomed Sci. 2017;9(3):113-8. PubMed | Google Scholar
- Tambyah PA, Koay ESC, Poon MLM, Lin RVTP, Ong BKC. Dengue Hemorrhagic Fever Transmitted by Blood Transfusion. New Engl J Med. 2008;359(14):1526-7. PubMed | Google Scholar
- Chuang V, Wong TY, Leung YH, Ma E, Law YL, Tsang O et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J. 2008;14(3):170-7. PubMed | Google Scholar
- Sabino EC, Loureiro P, Lopes ME, Capuani L, McClure C, Chowdhury D, Di-Lorenzo-Oliveira C, Oliveira LC, Linnen JM, Lee TH, Goncalez T, Brambilla D, Kleinman S, Busch MP, Custer B, International Component of the NRE, Donor Evaluation S, III. Transfusion-Transmitted Dengue and Associated Clinical Symptoms During the 2012 Epidemic in Brazil. J Infect Dis. 2016 Mar 1;213(5):694-702. PubMed | Google Scholar
- Ashshi AM. Serodetection of Dengue virus and its antibodies among blood donors in the western region of Saudi Arabia: a preliminary study. Blood Transfus. 2015 Jan;13(1):135-8. PubMed PMID: 25369603.. PubMed | Google Scholar
- Rodriguez Rodriguez D, Garza Rodriguez M, Chavarria AM, Ramos-Jimenez J, Rivera MA, Tamez RC et al. Dengue virus antibodies in blood donors from an endemic area. Transfus Med. 2009 Jun;19(3):125-31. PubMed | Google Scholar
- Ambrose JH, Sekaran SD, Azizan A. Dengue Virus NS1 Protein as a Diagnostic Marker: Commercially Available ELISA and Comparison to qRT-PCR and Serological Diagnostic Assays Currently Used by the State of Florida. J Trop Med. 2017:2017:8072491 PubMed | Google Scholar
- Kulkarni R, Tiraki D, Wani D, Mishra AC, Arankalle VA. Risk of transfusion-associated dengue: screening of blood donors from Pune, western India. Transfusion. 2019 Feb;59(2):458-62. PubMed | Google Scholar
- Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis. 2011 Aug;17(8):1349-54. PubMed | Google Scholar
- Tchuandom SB, Lissom A, Ateba GHM, Tchouangueu TF, Tchakounte C, Ayuk AR et al. Dengue virus serological markers among potential blood donors: an evidence of asymptomatic dengue virus transmission in Cameroon. Pan Afr Med J. 2020;36:185. PubMed | Google Scholar
- Vairo F, Nicastri E, Yussuf SM, Cannas A, Meschi S, Mahmoud MA et al. IgG against dengue virus in healthy blood donors, Zanzibar, Tanzania. Emerg Infect Dis. 2014 Mar;20(3):465-8. PubMed | Google Scholar
- Loua A, Nikiema JB, Sougou A, Kasilo OJM. Transfusion in the WHO African Region. Transfus Clin Biol. 2019 Sep;26(3):155-9. PubMed | Google Scholar
- Eichbaum Q, Shan H, Goncalez TT, Duits AJ, Knox P, Reilly J et al. Global health and transfusion medicine: education and training in developing countries. Transfusion. 2014 Jul;54(7):1893-8. PubMed | Google Scholar
- Arya SC, Agarwal N, Parikh SC, Agarwal S. Simultaneous Detection of Dengue NS1 Antigen, IgM plus IgG and Platelet Enumeration during an Outbreak. Sultan Qaboos Univ Med J. 2011 Nov;11(4):470-6. PubMed | Google Scholar
- Ashshi AM, Alghamdi S, El-Shemi AG, Almdani S, Refaat B, Mohamed AM et al. Seroprevalence of Asymptomatic Dengue Virus Infection and Its Antibodies Among Healthy/Eligible Saudi Blood Donors: Findings From Holy Makkah City. Virology (Auckl). 2017 Feb 24:8:1-5. PubMed | Google Scholar
- Konongoi L, Ofula V, Nyunja A, Owaka S, Koka H, Makio A et al. Detection of dengue virus serotypes 1, 2 and 3 in selected regions of Kenya: 2011-2014. Virol J. 2016 Nov 4;13(1):182. PubMed | Google Scholar
- Lutomiah J, Barrera R, Makio A, Mutisya J, Koka H, Owaka S et al. Dengue Outbreak in Mombasa City, Kenya, 2013-2014: Entomologic Investigations. PLoS Negl Trop Dis. 2016 Oct;10(10):e0004981. PubMed | Google Scholar
- Obonyo M, Fidhow A, Ofula V. Investigation of laboratory confirmed Dengue outbreak in North-eastern Kenya, 2011. PLoS One. 2018;13(6):e0198556. PubMed | Google Scholar
- Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019 Apr;13(Suppl 1):S31-s4.. PubMed | Google Scholar
- Calculator.net. Sample size calculator The Woodlands2023.
- Tapsoba ASA, Djigma FW, Bayala B, Sorgho PA, Traore L, Zohoncon TM et al. KIR2DL2, KIR2DL5A and KIR2DL5B Genes Induce Susceptibility to Dengue Virus Infection, while KIR3DL3 and KIR2DS5 Confer Protection. Mediterr J Hematol Infect Dis. 2022;14(1):e2022075. PubMed | Google Scholar
- Ellis EM, Neatherlin JC, Delorey M, Ochieng M, Mohamed AH, Mogeni DO et al. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis. 2015 Apr;9(4):e0003733. PubMed | Google Scholar
- Koech BJ, Seroprevalence of dengue fever virus in the adult Kenyan population In Nairobi, Eldoret and Kisumu Regions. 2015. Google Scholar
- Abd El-Wahab EW, Elfiky KSR, Ghanem MA, Shatat HZ. Assessment of dengue virus threat to blood safety and community health: A single center study in northern Egypt. J Virus Erad. 2022 Jun;8(2):100077. PubMed | Google Scholar
- Robinson M, Sweeney TE, Barouch-Bentov R, Sahoo MK, Kalesinskas L, Vallania F et al. A 20-Gene Set Predictive of Progression to Severe Dengue. Cell Reports. 2019;26(5):1104-11.e4. PubMed | Google Scholar
- Narkwa PW, Mutocheluh M, Kwofie TB, Owusu M, Annan A, Ali I, Boamah JK. Dengue virus exposure among blood donors in Ghana. Journal of Medical and Biomedical Sciences. 2016;5(2):30-5. PubMed | Google Scholar
- Lima M, de Lima RC, de Azeredo EL, Dos Santos FB. Analysis of a Routinely Used Commercial Anti-Chikungunya IgM ELISA Reveals Cross-Reactivities with Dengue in Brazil: A New Challenge for Differential Diagnosis. Diagnostics (Basel). 2021 Apr 30;11(5). PubMed | Google Scholar
- Ribas-Silva RC, Eid AA. Dengue antibodies in blood donors. Rev Bras Hematol Hemoter. 2012;34(3):193-5. PubMed | Google Scholar
- Slavov SN, Ciliao-Alves DC, Gonzaga FAC, Moura DR, de Moura A, de Noronha LAG et al. Dengue seroprevalence among asymptomatic blood donors during an epidemic outbreak in Central-West Brazil. PLoS One. 2019;14(3):e0213793. PubMed | Google Scholar
- Liao Q, Shan Z, Wang M, Huang J, Xu R, Huang K et al. An evaluation of asymptomatic Dengue infections among blood donors during the 2014 Dengue outbreak in Guangzhou, China. J Med Virol. 2017 Nov;89(11):2037-40. PubMed | Google Scholar
- Kwan TH, Lee SS, Chan DPC, Cheung M, Kam KM. Assessing the risk of dengue virus transmission in a non-endemic city surrounded by endemic and hyperendemic areas. Int J Infect Dis. 2017 Feb;55:99-101. PubMed | Google Scholar
- Li L, Li Y, Lu S, Dong J, Xu H, Zhang Q et al. Epidemiological survey and screening strategy for dengue virus in blood donors from Yunnan Province. BMC Infect Dis. 2021 Jan 22;21(1):104. PubMed | Google Scholar
- Swedan S, Al-Saleh D. Transfusion transmitted virus and dengue virus among healthy blood donors: A prevalence report from Jordan. Biomol Biomed. 2023 May 1;23(3):450-6. PubMed | Google Scholar
- Jain A, Jain S, Chowdhury N. Seroprevalence of dengue in blood donors in an outbreak: experience of a blood bank in north India. Trop Doct. 2019 Jul;49(3):212-5. PubMed | Google Scholar
- Raj ARR, Shashindran N, Shenoy V, Kumar A. Dengue seropositivity among blood donors in a tertiary hospital in Kerala, Southern India. Ann Afr Med. 2022 Jan-Mar;21(1):39-42. PubMed | Google Scholar
- Remakanth R, Basavarajegowda A, Dhodapkar R. Prevalence of dengue NS1 antigenemia among healthy blood donors in a tertiary care hospital in Southern India. Asian J Transfus Sci. 2021 Jul-Dec;15(2):140-5. PubMed | Google Scholar
- Gao Z, Zhang Y, Yang Y, Xu M, Liao P, He W et al. Dengue virus infections among blood donors in Guangxi of China, 2013-2014. Transfus Med. 2018 Jun;28(3):236-42. PubMed | Google Scholar
- Singh MP, Majumdar M, Singh G, Goyal K, Preet K, Sarwal A et al. NS1 antigen as an early diagnostic marker in dengue: report from India. Diagn Microbiol Infect Dis. 2010 Sep;68(1):50-4. PubMed | Google Scholar
- Mahapatra D, Sarangi G, Mahapatra A, Paty BP, Das P, Chayani N. NS1 Antigen Capture ELISA an Effective Method for Diagnosis of Early Dengue Infection - Report of an Outbreak at Angul District, Odisha, India. J Clin Diagn Res. 2014 Aug;8(8):Dc08-10. PubMed | Google Scholar
- Nunes PCG, Nogueira RMR, Heringer M, Chouin-Carneiro T, Damasceno Dos Santos Rodrigues C, de Filippis AMB, Lima M, Dos Santos FB. NS1 Antigenemia and Viraemia Load: Potential Markers of Progression to Dengue Fatal Outcome? Viruses. 2018 Jun 14;10(6):326. PubMed | Google Scholar
- Pereira SS, Andreata-Santos R, Pereira LR, Soares CP, Félix AC, de Andrade P et al. NS1-based ELISA test efficiently detects dengue infections without cross-reactivity with Zika virus. Int J Infect Dis. 2021 Nov;112:202-4. PubMed | Google Scholar