Seroprofile of leptospiral antibodies and a simple tube-elisa for serological evaluation of antibodies to leptospira in dogs
Godspower Obokparo Ohore, Theophilus Aghogho Jarikre, Benjamin Obukowho Emikpe, Derrick Adu Asare
Corresponding author: Benjamin Obukowho Emikpe, Department of Pathobiology, School of Veterinary Medicine, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana 
Received: 28 Dec 2023 - Accepted: 31 Mar 2024 - Published: 17 Apr 2024
Domain: Laboratory medicine,Public health,Zoology
Keywords: Leptospirosis, antibodies, serology, sero-profile, dogs, Nigeria
©Godspower Obokparo Ohore et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Godspower Obokparo Ohore et al. Seroprofile of leptospiral antibodies and a simple tube-elisa for serological evaluation of antibodies to leptospira in dogs. PAMJ-One Health. 2024;13:15. [doi: 10.11604/pamj-oh.2024.13.15.42530]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/13/15/full
Research 
Seroprofile of leptospiral antibodies and a simple tube-elisa for serological evaluation of antibodies to leptospira in dogs
Seroprofile of leptospiral antibodies and a simple tube-elisa for serological evaluation of antibodies to leptospira in dogs
![]() Godspower Obokparo Ohore1, Theophilus Aghogho Jarikre1,
Godspower Obokparo Ohore1, Theophilus Aghogho Jarikre1, ![]() Benjamin Obukowho Emikpe2,&,
Benjamin Obukowho Emikpe2,&, ![]() Derrick Adu Asare2
Derrick Adu Asare2
&Corresponding author
Introduction: leptospirosis is a contagious disease affecting both humans and domestic animals posing a significant global public health threat. This study examined leptospirosis vaccination adherence, and sero-profile of leptospiral antibodies in dogs using a novel tube-ELISA method to overcome existing diagnostic limitations.
Methods: blood samples from 134 dogs in Southwest Nigeria were collected, and their vaccination status, breed, sex, and age were documented. A plate ELISA served as a reference method, while a tube-ELISA was developed for individual samples and compared to the plate-ELISA. Data analysis utilized chi-square and Fisher exact tests.
Results: the study revealed a 77.6% adherence to leptospirosis vaccination among sampled dogs, varying among breeds. Notably, 41.8% of dogs had detectable leptospiral antibodies, and 40% of unvaccinated dogs were seropositive, underscoring their role as potential pathogen carriers. Post-vaccination seropositivity rates varied over time intervals without significant differences. The tube-ELISA exhibited 83.3% sensitivity and 100% specificity compared to the plate-ELISA, suggesting its diagnostic potential.
Conclusion: this study underscores the challenges in controlling leptospirosis, including low vaccination adherence and limited seroconversion rates among vaccinates. The tube-ELISA offers a cost-effective method for individual dog sero-monitoring, mitigating existing diagnostic constraints.
Leptospirosis is a contagious zoonotic disease of ubiquitous distribution that affects most domestic animals including dogs as well as humans [1]. It is historically known to be transmitted through contact with infected materials such as urine [2] and aborted fetuses from affected animals. The disease is also therefore an important zoonosis that endangers the health of man and warm-blooded animals in many parts of the world with clinical presentations that range from acute renal failure to hepatocellular injury [3].
Dogs are susceptible to all known Leptospira serovars depending on the prevailing epidemiological situation [4], but the predominant serotypes in dogs are L. canicola and L. icterohaemorrhagiae [5]. Several reports show that leptospirosis is an endemic disease in dogs in Nigeria [6]. Although control of the disease in dogs is mainly through vaccination, it has been observed that vaccinated dogs can still succumb to infections especially when such infection is caused by heterologous strains different from vaccinal strains because cross-protection between strains is at best partial [7].
Although the need to monitor the immune status of vaccinated and unvaccinated dogs to establish the susceptibility of these companion animals to the leptospiral agent has become paramount, the cost and ease of achieving this with available diagnostic tests remain a challenge in routine clinical practice, especially in resource-limited settings [8]. Microscopic agglutination test (MAT), regarded as the diagnostic 'gold standard' is cumbersome, time-consuming, and potentially hazardous due to the requirement for maintenance of live leptospirae cultures, while the standard plate ELISA though ideal for mass testing, is not sufficient for testing individual samples due to preset reagent requirements and contamination of unused wells in test plates [8]. Snap disk tests have recently become available for qualitative diagnosis for individual samples but are not readily available and relatively expensive to use in many routine clinical settings.
There is, therefore, a need for the development of a complementary simple and low-cost test that is suited for testing individual samples and requiring minimal instrumentation and equipment, for the clinical evaluation of individual dogs both for post-vaccination responses and in paired sample evaluation of suspected leptospirosis cases in a clinical setup, especially in resource-poor settings. This report presents a simple in-house diagnostic tube-ELISA useful for the serological evaluation of leptospiral antibodies in an individual dog sample as may be encountered in routine clinical practice and the sero-profile of leptospiral antibodies in companion dogs in Southwest Nigeria.
Study design and study area: this study is a cross-sectional study which was conducted in two major Government-owned veterinary clinics and three private veterinary centers in the Ibadan metropolis in Oyo State as well as a dog kennel maintained in Abeokuta, Ogun state in Nigeria.
Dog sera collection: blood samples were collected via cephalic venipuncture from a total of one hundred and thirty-four (134) different breeds of dogs presented to two major Government-owned veterinary clinics and three private veterinary centers in the Ibadan metropolis as well as a dog kennel maintained by a government paramilitary parastatal in Abeokuta, Ogun state. Sera were separated from clotted blood samples and stored at -25°C until analyzed. Clinical records of leptospira vaccination status and the post-vaccination interval before sample collection, breed, sex, and age of the dogs were obtained from the clinical or the kennel records of the dogs as applicable. Clinical records of sampled dogs were identified and flagged to avoid repeated sampling.
Plate enzyme-linked immunosorbent assay for leptospiral antibodies: the antibody detection plate enzyme-linked immunosorbent assay was conducted by modification of the method described previously [9]. Briefly, flat bottom micro-ELISA plates were coated with 100µl (approximately 5µg) of a tri-valent (L. icterohaemorrhagiae, L. canicola, L. grippotyphosa) vaccine antigen (Biocan®, Bioveta, a.s, Czech Republic) diluted in carbonate buffer pH 9.6. The plates were incubated overnight at 4°C. Thereafter, excess coating antigen was washed off with phosphate-buffered saline containing 0.5% Tween 20 (PBST). A 100µl of 1:50 dilution of test and control sera in PBST was added to duplicate wells. The plates were incubated at room temperature for 30 minutes and were intermittently shaken to mix the sample solutions for an optimized antigen-antibody interface.
Plates were washed 3 times in PBST as previously described and then 100µl of rabbit anti-dog horseradish peroxidase conjugate® (Sigma, USA) diluted 1:1000 in PBST was dispensed into each well. The plates were incubated at room temperature for 30 minutes. The excess conjugate was washed off in three washes with PBST. All dilutions were pre-determined through several checkerboard titrations. Thereafter, 100µl of chromogenic substrate solution comprising of tetramethylbenzidine (TMB) HCL (Sigma® USA) and hydrogen peroxide substrate was added to each well. The plates were incubated in the dark for 15 minutes and then observed for colour development. The optical densities (OD) of the wells were read off an ELISA plate reader at 450nm wavelength. The sample-to-positive ratios (SPR) for each sample were calculated as:
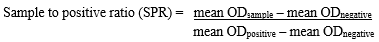
The cut-off SPR of 0.450, equivalent to the mean OD +4SD as suggested by Ribotta et al. [9] of the negative control was used.
Tube enzyme-linked immunosorbent assay: the tube ELISA was adapted from the conventional plate ELISA protocol with some modifications. In this protocol, a one-milliliter polystyrene tube (Sigma® U.K) was coated with approximately 0.5mls/tube of approximately 10µg/ml of trivalent leptospira vaccine antigen (Biocan® Bioveta, a. s, Czech Republic), consisting of L. icterohaemorrhagiae, L. canicola and L. grippotyphosa. The tubes were sealed with Parafim M®, kept at room temperature for about 30 minutes with intermittent mixing, and thereafter incubated at 4oC at least overnight prior to use.
In carrying out the assay, the excess coating antigen was simply discarded and the tubes were rinsed once by the addition of 10 drops of PBST into each tube using a Pasteur pipette. The tubes were allowed to stand for about 2 minutes and the washing buffer was then discarded. Nine drops of PBST with 5% skimmed milk were added to each tube followed by 1 drop of test or control serum (1:10) in respective tubes. The tubes were incubated at RT for 45 minutes with intermittent mixing. Excess reactants were discarded and the tubes were washed twice with 10 drops of PBST each as described above. Thereafter, 5 drops of pre-diluted rabbit anti-dog HRPO conjugate (Sigma® USA) was added to each tube and gently mixed and incubated for 30 mins. The tubes were washed as previously stated and then 6 drops of a commercial ready-to-use substrate and chromogen (ProCheck®, Switzerland) were added to each tube and again gently mixed and incubated at RT in a dark enclosure of a laboratory cabinet. The colour development was visually observed after 30 minutes. The performance of the tube-ELISA relative to the plate-ELISA was further evaluated using 36 purposively selected samples previously tested with the plate-ELISA. The relative diagnostic sensitivity and specificity of the tube-ELISA regarding the plate-ELISA were calculated from the results obtained as described by Ribotta et al. [9].
Data analysis: the data obtained were presented in the form of tables and analyzed using the Chi-square test and the Fisher exact test with the aid of the Statistical Package for Social Sciences (SPSS) software version 17. Values of p < 0.05 were considered statistically significant. Descriptive analysis was done using percentages. The diagnostic sensitivity and specificity of the tube-ELISA relative to the plate-ELISA were calculated based on the method described by Ribotta et al. [9]. Sensitivity was calculated as:
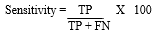
Where TP is the number of sera positive by plate- and tube-ELISA and FN is the number of sera positive detected by plate-ELISA but negative by tube-ELISA. Specificity was calculated as:
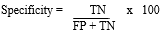
Where TN is the number of sera negative by plate ELISA and tube ELISA and FP is the number of negative sera detected by plate ELISA but positive by tube ELISA.
Ethical considerations: informed consent of the owners of dogs from which the blood samples were obtained after the objectives of the study and the procedures involved were fully explained. The blood sample collection from the study animals was performed according to the World Organization for Animal Health (WOAH) standards and guidelines for blood collection from animals for cases of Leptospirosis [10].
Leptospirosis vaccination adherence in dogs: the relative dog owner´s adherence to leptospirosis vaccination and the prevalence of antibodies in vaccinated and unvaccinated dogs sampled in this study are shown in Table 1. An overall 77.6% adherence to leptospirosis vaccination was observed among the dog population sampled in this study, while 22.4% of the dogs were unvaccinated against leptospirosis. Among the sampled dog population only 56 out of the 134 dogs (41.8%) had detectable antibodies to leptospira, indicating a relatively low modest population seroconversion in the dogs. There was a significant difference (p<0.05) in vaccination adherence between different breeds of dogs. The lowest vaccination adherence was observed in boxers and local dogs, with a respective 33.3% and 44.4% adherence to vaccination against leptospirosis. On the other hand, 80.6% of the most popular breed, Alsatian were vaccinated, while 100% vaccination adherence was observed in Labradors and other exotic but less popular breeds (Ridgeback, Caucasian, and Dobberman). Among the unvaccinated breeds, all 10 (100%) unvaccinated local dogs sampled in this study were positive for leptospiral antibodies, while 2 of 14 (14.3%) unvaccinated Alsatian and none of the other unvaccinated exotic breeds had detectable antibodies to leptospira. There was a significant difference in positive seroprevalence between unvaccinated breeds of dogs (Table 1). However, there was no significant difference (p>0.05) in the immune responses of the vaccinated dogs based on breed. Similarly, there was no significant difference (p>0.05) in the responses to vaccination based on sex (Table 2).
Prevalence of leptospiral antibodies in vaccinated and unvaccinated dogs: analysis of the seroconversion rate with reference to active vaccination status also showed that 44 out of the 104 dogs (42.3%) that received leptospirosis vaccination at different post-vaccination periods had varying levels of detectable anti-leptospira antibodies. Similarly, 12 out of 30 unvaccinated dogs (40.0%) also had varying levels of antibodies that ranged from low to very high sample to positive ratios (Table 3). Post-vaccination chronological analysis showed detectable antibodies in 53.3% of dogs at 0 to 3 months post-vaccination; 46.2% at >3 to 6 months; 35.7% at >6 to 9 months; 42.9% at >9 to 12 months and 0% above 12 months post-vaccination (Table 3). There was no significant difference (p>0.05) in the mean levels of antibodies to leptospiral vaccination between post-vaccination time intervals and the seropositivity rates detected in the dog population.
Performance of tube-ELISA with reference to microplate-ELISA: the comparative results of the tube-ELISA with reference to microplate-ELISA in leptospiral antibody detection in dogs are shown in Table 4. The relative sensitivity and specificity of the tube-ELISA with reference to microplate-ELISA in the detection of anti-leptospiral antibodies in the dog were found to be 83.3% and 100% respectively, while the predictive values of negative and positive samples respectively were 50% and 100%, indicating a good correlation/agreement between the simple tube-ELISA test and microplate-ELISA. However, 6 out of 30 (20%) of the dogs that tested positive (low) by microplate-ELISA showed no detectable antibodies by the tube-ELISA, but interestingly, all 3 negative samples showed perfect agreement with both methods. A visual result of the tube-ELISA test is shown in Figure 1. Tubes labeled - and + are negative and positive controls respectively; tubes 1-7 are test samples with varying results from negative (tubes 1 to 3) to positive (tubes 4 to 7) reactions to leptospiral antibodies. Tube 6 shows a very high positive with a colour change from blue to light brown at the read point.
This study describes the leptospirosis vaccination adherence and relative seroprevalence of leptospiral antibodies in dogs presented to two major government-owned veterinary clinics and three private clinics, including a south-west Nigerian private kennel, and the development of a simple tube-ELISA sero-diagnostic/monitoring tool. This study vaccinated 77% of dogs in veterinary clinics and private kennels against leptospirosis. Leptospirosis is a major zoonosis, but Nigeria has an ambiguous vaccination policy. Adeniyi Okewole and Oluremi Ayoola [11] found 98% leptospirosis vaccination adherence in 52 dogs from three Ibadan and Lagos clinics. Leptospirosis vaccination adherence was 77%, lower than Adeniyi Okewole and Oluremi Ayoola [11] found in the same Nigerian region and suboptimal for dog disease control. Unvaccinated dogs can spread the disease to other susceptible dogs and animals by acting as carriers/reservoirs (shedders) of the organism. Most Leptospira serovars infect different animals, but each has a primary host reservoir to survive and spread [12]. Dogs maintain serovar canicola and host others incidentally [12]. This is even more concerning because the dogs in this study were owned by people who took them to the vet. The true population vaccination rate is likely much lower than 77% because scavenging, free-roaming dogs are less likely to be vaccinated. Previous studies show that vaccinated and infected dogs shed the organism in their urine without clinical disease. In this study, breed and owners' dog value affected vaccination adherence. The local dog, which is low-value and kept on a partial range system, had 44.4% vaccination adherence. In Ibadan, Agunloye et al. [13] found 9% leptospirosis vaccination adherence for this breed of dogs. Low vaccination adherence in boxers may be due to the belief that indoor pets are immune to infections and do not need protective vaccination. No data in this study supports this opinion.
This study found antibodies in 100% of unvaccinated local dogs, which may be due to the smaller sample size, the higher sensitivity of the ELISA method compared to the MAT as reported by Bajani et al. [14], and an increasing rate of environmental leptospira exposure and infection. Agunloye et al. [13] found 14.4% leptospirosis in unvaccinated local dogs at two Ibadan veterinary clinics using MAT. Epidemiological risk is shown by unvaccinated dogs' high leptospirosis rate. In a partial or absolute roaming management system, most local dogs may be exposed, infected, or carriers of leptospirae. Nigerian studies found leptospirae in 20-80% of unvaccinated dogs [15]. In another Japanese study, Iwamoto et al. [16] found antibodies against Leptospira icterohaemorrhagiae in healthy unvaccinated dogs. Other studies found asymptomatic leptospire carriers in dogs that recovered from acute and subclinical infections [17] and natural infections in unvaccinated but seropositive dogs [18]. Interestingly, no unvaccinated boxer or Pitbull seroconverted to leptospira. While leptospiral organism prevention is unlikely [18], this finding suggests that dogs can be protected from infectious sources with proper biosecurity. This suggests that high-valued pet breeds may have low vaccination adherence due to a low infection risk.
In this study, only 42.3% of vaccinated dogs seroconverted to leptospira. Vaccinated dogs in South-west Nigeria may have high leptospirosis rates due to low seroconversion. Nigeria's poor power, cold chain, and vaccine storage systems may explain low seroconversion rates in vaccinated dogs. Since cross-protection is partial, Adeniyi Okewole and Oluremi Ayoola [11] suggest other leptospira serovars may infect vaccinated animals. Because vaccination induces serovar-specific immunity, OIE [10] recommends that vaccines for a specific animal species in a specific region contain only leptospira serovars and genotypes that cause problems or are transmitted by the animal. Andre-Fontaine [19] says dog leptospirosis vaccines are inadequate. MAT found infection in 36.2% of vaccinated dogs and 13.7% probable. Several proprietary vaccines cause patent leptospiraemia and urine shedding after challenge [7]. South-west Nigerian dogs' prevalent serovars have no local vaccines, and recent studies have been inadequate [11].
The absence of a significant difference between the post-vaccination time interval and the detectable level or seropositivity rate of leptospiral antibodies in dogs in south-west Nigeria suggests unpredictable immune responses to various leptospira vaccines or modulation of immune responses by both vaccinal immunity and periodic field exposure to leptospiral infection. Unvaccinated dogs exhibit a 40% seropositive prevalence, indicating a high dog exposure rate. Schuller et al. [20] reported that acquired immunity after vaccination may last only around 6 months, advocating for biannual boosts in high-risk dogs. The low seropositive rate at different post-vaccination periods in this study implies a potentially inadequate seroconversion rate to Nigeria's leptospiral vaccines, contributing to poor protection and disease control challenges in vaccinated dogs [21].
This study introduces the first use of tube-ELISA for dog leptospiral antibodies, a method similar to that employed for trypanosomiasis diagnosis in humans and animals [22]. While MAT remains the gold standard for leptospirosis diagnosis, its complexity and requirement for live cultures make it impractical. Plate-ELISA is suitable for large sample analyses but not for individual case testing [23,24]. The tube-ELISA method developed in this study, akin to the Snap, is tailored for individual serodiagnosis, reducing reagent waste and cost. In a limited sample assay, tube-ELISA demonstrated 83.3% sensitivity and 100% specificity over microplate-ELISA, indicating its potential use in clinical settings for leptospirosis diagnosis and vaccination response assessment, capturing long-lasting IgG antibodies. It can be particularly valuable in paired sample analysis without the need for extensive testing or the risk of unused good contamination associated with plate-ELISA. However, subjective interpretation may impact results over time, mitigated by spectrophotometric measurement. The tube-ELISA's 50% average predictive value for negative samples was lower than plate-ELISA, but its accuracy in predicting positive samples was comparable.
Many ELISAs, often validated against MAT with less than 50% sensitivity in chronic infections, face challenges in validation. This preliminary report on a small sample underscores the necessity for further studies to determine the tube-ELISA's sensitivity and specificity compared to MAT or sequential sera from culture-positive cases for comprehensive clinical diagnosis of leptospirosis in dogs. While not designed for large-scale epidemiological surveys due to individual tubes, a comparative field trial can reveal the tube-ELISA's potential in leptospiral serology diagnostics and sero-profiling in dogs. To enhance objectivity in low-level clinical laboratories, additional tests are required to establish a standard color band for qualitative interpretation of the tube-ELISA's colored end-point.
Controlling leptospirosis in dogs in southwest Nigeria is challenging due to low vaccination adherence, unpredictable seroconversion among vaccinates, and potential exposure from the environment, seen in high seroconversion rates in local unvaccinated dogs. The absence of simple, inexpensive diagnostic tests complicates clinical evaluation. To address this, increased awareness for vaccination adherence and post-vaccination seroconversion evaluation is crucial. The tube-ELISA method developed in this study facilitates individual dog serological evaluation, offering advantages over plate-ELISA, which is economically unrealistic for individual cases. Despite efforts to control canine leptospirosis through vaccination in Nigeria, success has been partial due to factors like poor knowledge of emerging serovars, imported vaccines potentially preventing only clinical disease, increasing numbers of "carrier" dogs shedding contagion, mismatched vaccine serovars, poor vaccine storage, and a lack of simple routine diagnostic assays. A comprehensive national study of prevalent Leptospira serovars in the animal population is recommended to inform the development of a local vaccine for effective use in Nigeria.
What is known about this topic
- Leptospirosis poses challenges globally as a zoonotic disease affecting humans and animals, with a historical emphasis on its impact on dogs;
- Existing literature recognizes challenges in leptospirosis control, particularly related to achieving high vaccination adherence and ensuring consistent seroconversion in vaccinated dogs;
- Traditional diagnostic methods, such as the microscopic agglutination test (MAT), are acknowledged for their complexity, time-consuming nature, and potential hazards, highlighting the need for simpler and more cost-effective diagnostic alternatives.
What this study adds
- The study contributes localized insights by assessing leptospirosis vaccination adherence and seroconversion rates specifically in southwest Nigeria;
- The study introduces a cost-effective tube-ELISA as a practical alternative for individual serodiagnosis. This novel diagnostic tool addresses the limitations of traditional methods, providing a more accessible option, particularly in resource-limited settings;
- The study offers breed-specific insights into vaccination adherence and seroprevalence. This detailed analysis provides a nuanced understanding of leptospirosis dynamics, allowing for more targeted and effective vaccination strategies in different dog breeds in southwest Nigeria.
The authors declare no competing interests.
Godspower Obokparo Ohore and Theophilus Aghogho Jarikrem conceptualized, designed the research work, acquired data, analyzed data and drafted the initial manuscript. Benjamin Obukowho Emikpe reviewed the draft and final manuscript critically for important intellectual content. Derrick Adu Asare reviewed and drafted the final manuscript. All the authors have read and agreed to the final manuscript.
The authors would like to appreciate the management of the 2 major Government-owned veterinary clinics and three private veterinary centers in the Ibadan metropolis in Oyo State as well as a dog kennel maintained in Abeokuta, Ogun state in Nigeria for being available for sample collection for this study.
Table 1: anti-leptospira antibody seroconversion rate in vaccinated and unvaccinated dogs presented to veterinary clinics
Table 2: anti-leptospira antibody seroconversion rate in dogs at various month intervals post-vaccination
Table 3: comparative seroconversion rate to leptospirosis based on sex in dogs
Table 4: diagnostic performance of tube-ELISA concerning microplate-ELISA in detection of leptospiral antibodies
Figure 1: diagnostic performance of tube-ELISA concerning microplate-ELISA in detection of leptospiral antibodies
- Pijnacker R, Goris MG, Te Wierik MJ, Broens EM, van der Giessen JW, de Rosa M et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Euro Surveill. 2016 Apr 28;21(17). PubMed | Google Scholar
- Haake DA, Levett PN. Leptospirosis in humans. Leptospira and leptospirosis. Curr Top Microbiol Immunol. 2015;387:65-97. PubMed | Google Scholar
- Sohail ML, Khan MS, Ijaz M, Naseer O, Fatima Z, Ahmad AS et al. Seroprevalence and risk factor analysis of human leptospirosis in distinct climatic regions of Pakistan. Acta Trop. 2018 May;181:79-83. PubMed | Google Scholar
- Arent ZJ, Andrews S, Adamama-Moraitou K, Gilmore C, Pardali D, Ellis WA. Emergence of novel Leptospira serovars: a need for adjusting vaccination policies for dogs? Epidemiol Infect. 2013 Jun;141(6):1148-53. PubMed | Google Scholar
- Morikawa VM, Bier D, Pellizzaro M, Ullmann LS, Paploski IA, Kikuti M et al. Seroprevalence and seroincidence of Leptospira infection in dogs during a one-year period in an endemic urban area in Southern Brazil. Rev Soc Bras Med Trop. 2015 Jan-Feb;48(1):50-5. PubMed | Google Scholar
- Pilau NN, Lubar AA, Daneji AI, Mera UM, Magaji AA, Abiayi EA et al. Serological and molecular epidemiology of leptospirosis and the role of dogs as sentinel for human infection in Nigeria. Heliyon. 2022 May 21;8(5):e09484. PubMed | Google Scholar
- Sant´Anna da Costa R, Di Azevedo MI, dos Santos Baptista Borges AL, Aymée L, Martins G, Lilenbaum W. Effect of Vaccination against Leptospira on Shelter Asymptomatic Dogs Following a Long-Term Study. Animals (Basel). 2022 Jul 12;12(14):1788. PubMed | Google Scholar
- Goris MG, Hartskeerl RA. Leptospirosis serodiagnosis by the microscopic agglutination test. Curr Protoc Microbiol. 2014 Feb 6:32:Unit 12E.5. PubMed | Google Scholar
- Ribotta MJ, Higgins R, Gottschalk M, Lallier R. Development of an indirect enzyme-linked immunosorbent assay for the detection of leptospiral antibodies in dogs. Can J Vet Res. 2000 Jan;64(1):32-7. PubMed | Google Scholar
- WOAH. Leptospirosis. Terrestrial Manual. 2021.
- Adeniyi Okewole E, Oluremi Ayoola M. Seroprevalence of leptospiral serovars other than Canicola and Icterohaemorrhagiae in dogs in the Southwestern Nigeria. Veterinarski arhiv. 2009 Feb 24;79(1):87-96. Google Scholar
- Chadsuthi S, Bicout DJ, Wiratsudakul A, Suwancharoen D, Petkanchanapong W, Modchang C et al. Investigation on predominant Leptospira serovars and its distribution in humans and livestock in Thailand, 2010-2015. PLoS Negl Trop Dis. 2017 Feb 9;11(2):e0005228. PubMed | Google Scholar
- Agunloye CA. Leptospiral aggutinating antibodies in sheep and goats in South-West Nigeria. Israel Journal of Veterinary Medicine. 2002;57(1):28-30. Google Scholar
- Bajani MD, Ashford DA, Bragg SL, Woods CW, Aye T, Spiegel RA et al. Evaluation of four commercially available rapid serologic tests for diagnosis of leptospirosis. J Clin Microbiol. 2003 Feb;41(2):803-9. PubMed | Google Scholar
- Agunloye CA, Alabi FO, Olaleye OD. Leptospirosis in nigerian: a seroepidemiological survey. Indian Veterinary Journal (India). 2001;78(5). Google Scholar
- Iwamoto E, Wada Y, Fujisaki Y, Umeki S, Jones MY, Mizuno T et al. Nationwide survey of Leptospira antibodies in dogs in Japan: results from microscopic agglutination test and enzyme-linked immunosorbent assay. J Vet Med Sci. 2009 Sep;71(9):1191-9. PubMed | Google Scholar
- Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A et al. Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009 Jan 15;199(2):159-67. PubMed | Google Scholar
- Grippi F, Blanda V, Galluzzo P, Bongiorno M, Sciacca C, Arcuri F et al. A Canine Leptospirosis Clinical Case Due to Leptospira interrogans (Serogroup Icterohaemorrhagiae) in a Dog Kennel in Castelvetrano (Western Sicily, South Italy). Veterinary Sciences. 2023 Aug 6;10(8):508. Google Scholar
- Andre-Fontaine G. Diagnosis algorithm for leptospirosis in dogs: disease and vaccination effects on the serological results. Vet Rec. 2013 May 11;172(19):502. PubMed | Google Scholar
- Schuller S, Francey T, Hartmann K, Hugonnard M, Kohn B, Nally JE et al. European consensus statement on leptospirosis in dogs and cats. J Small Anim Pract. 2015 Mar;56(3):159-79. PubMed | Google Scholar
- Miotto BA, Guilloux AG, Tozzi BF, Moreno LZ, da Hora AS, Dias RA et al. Prospective study of canine leptospirosis in shelter and stray dog populations: Identification of chronic carriers and different Leptospira species infecting dogs. PLoS One. 2018 Jul 11;13(7):e0200384. PubMed | Google Scholar
- Chappuis F, Loutan L, Simarro P, Lejon V, Buüscher P. Options for field diagnosis of human African trypanosomiasis. Clin Microbiol Rev. 2005 Jan;18(1):133-46. PubMed | Google Scholar
- Goris M, Leeflang M, Boer K, Goeijenbier M, van Gorp E, Wagenaar J et al. Establishment of valid laboratory case definition for human leptospirosis. Journal of Bacteriology & Parasitology. 2011 Nov 25;3(2). Google Scholar
- Agampodi SB, Dahanayaka NJ, Nöckler K, Anne MS, Vinetz JM. Redefining gold standard testing for diagnosing leptospirosis: further evidence from a well-characterized, flood-related outbreak in Sri Lanka. Am J Trop Med Hyg. 2016 Sep 7;95(3):531-536. PubMed | Google Scholar





