Urinary schistosomiasis outbreak in Cabo Verde: the first description in the country, May-June, 2022
Veruska Maia da Costa, Menilita Paula Varela dos Santos Barbosa, Isaias Baptista Fernandes Varela, Aniceto Tavares dos Santos, Jonas Antônio Lopes Gomes, Graça Maria Carvalho Mendes Moniz, Ludmila dos Santos Miranda, Jaelsa Mira Gonçalves Moreira, Liliane Margareth Teixeira Hungria Silva, Jonas Lotufo Brant de Carvalho, Maria da Luz Lima Mendonça
Corresponding author: Veruska Maia da Costa, Cabo Verde Field Epidemiology Training Program, National Institute of Public Health, Ministry of Health, Praia, Santiago Island, Cabo Verde 
Received: 13 Aug 2023 - Accepted: 18 Mar 2024 - Published: 15 Apr 2024
Domain: Epidemiology, Infectious diseases epidemiology, Public health
Keywords: Schistosoma haematobium, outbreak investigation, public health
©Veruska Maia da Costa et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Veruska Maia da Costa et al. Urinary schistosomiasis outbreak in Cabo Verde: the first description in the country, May-June, 2022. PAMJ-One Health. 2024;13:13. [doi: 10.11604/pamj-oh.2024.13.13.41435]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/13/13/full
Outbreak investigation 
Urinary schistosomiasis outbreak in Cabo Verde: the first description in the country, May-June, 2022
Urinary schistosomiasis outbreak in Cabo Verde: the first description in the country, May-June, 2022
![]() Veruska Maia da Costa1,2,&, Menilita Paula Varela dos Santos Barbosa1,3,
Veruska Maia da Costa1,2,&, Menilita Paula Varela dos Santos Barbosa1,3, ![]() Isaias Baptista Fernandes Varela1,3, Aniceto Tavares dos Santos1,4, Jonas Antônio Lopes Gomes1,3, Graça Maria Carvalho Mendes Moniz1,5, Ludmila dos Santos Miranda1,6, Jaelsa Mira Gonçalves Moreira1,7, Liliane Margareth Teixeira Hungria Silva1,8, Jonas Lotufo Brant de Carvalho2,
Isaias Baptista Fernandes Varela1,3, Aniceto Tavares dos Santos1,4, Jonas Antônio Lopes Gomes1,3, Graça Maria Carvalho Mendes Moniz1,5, Ludmila dos Santos Miranda1,6, Jaelsa Mira Gonçalves Moreira1,7, Liliane Margareth Teixeira Hungria Silva1,8, Jonas Lotufo Brant de Carvalho2, ![]() Maria da Luz Lima Mendonça1,3
Maria da Luz Lima Mendonça1,3
&Corresponding author
Schistosomiasis is a public health problem with economic consequences in endemic areas. Cabo Verde is an insular country with no registry of schistosomiasis. In May 2022, a patient was reported with S. haematobium in urinalysis. An investigation was conducted to confirm diagnostic and outbreak, transmission sources to prevent additional illness. Two studies were conducted. First, using the snowball approach, starting with the first case identified. Second, using retrospective medical records. Suspected, confirmed, and discarded case definitions for urinary schistosomiasis (SCHu) were adopted. Interviews and urine samples were collected. An environmental investigation was conducted to identify the host snail. Identified 10 suspected cases, 8 (72.7%) were confirmed, the median age of 15 years (range: 7-24), all male with hematuria, living in a small community. In Schu's cases interviewed, 4(57.1%) walked barefoot in water and 3(75%) swam in water tanks. In a retrospective study, 28,702 medical records were evaluated, from which 49 suspected SCHu cases, 23(47%) interviewed, 19(82.6%) negatives, and 4(17.4%) did not collect samples. This is the first schistosomiasis record in Cabo Verde. The infection mechanism remains unknown as the specific reservoir was not found (Bulinus). We recommend epidemiological inspections and environmental assessment in communities to elucidate SCHu source transmission.
Schistosomiasis (SCH) is one of the neglected tropical diseases, especially in poor communities without access to potable water and adequate sanitation which poses serious public health consequences around the world, particularly on the African continent [1-3]. The disease is caused by trematodes of the genus Schistosoma and the transmission occurs when people come in contact with cercariae-infested water bodies [4,5] and it is more common in poor rural communities. Domestic activities such as washing clothes and fetching water in infected water, expose women and children to infection. Recreational activities like swimming and poor hygiene also make children vulnerable to schistosomiasis [1-3,6]. Humans are usually infected by five species of schistosomes, namely Schistosoma mansoni, Schistosoma haematobium, Schistosoma japonicum, Schistosoma mekongi and Schistosoma intercalatum [4]. In four of the five species, the adult worms live in the capillary network around the human intestines where they release thousands of eggs daily many of which end up in the liver, thereby causing intestinal schistosomiasis. S. haematobium, being the exception, causes the urinary form of the disease (SCHu) due to its location around the bladder where the inflammatory reaction to the eggs not only affects the bladder but also the urethra, ureters, and kidney and the principal clinical presentation is hematuria and dysuria [7,8]. S. haematobium infection is a major and yet poorly studied cause of chronic kidney disease in Africa [7,9,10].
The snail hosts are crucial for determining the range of schistosomiasis and are responsible for the focal nature of the disease. The risk of infection is dependent on seasonal changes in snail populations, water levels, infection rates, and cercarial output. Flooding events may also cause temporarily higher rates of infection in human communities. Information on snail hosts and the distribution of cercariae are important tools in the control and elimination of schistosomiasis. Bulinid snails are the most common hosts for human blood fluke Schistosoma haematobium, responsible for approximately two-thirds of the estimated 237 million cases of schistosomiasis [11].
Cabo Verde is a country located in West Africa and until then there were no case reports of schistosomiasis in humans, but records as well as the presence of the host of official animals in the archipelago [12,13]. The National Institute of Public Health of Cabo Verde (INSP) is the agency responsible for public health surveillance in the country, as well as training professionals in the field of epidemiology and supporting outbreak investigations. On May 5, a patient was reported to INSP with the presence of S. haematobium eggs on urinalysis. Thus, the objective of this investigation was to confirm the diagnosis and the outbreak; sources of transmission; ways to prevent additional illness, and propose infection prevention and control measures.
Study design: we conducted the outbreak investigation during the 10 May and 7 June 2022, through two descriptive studies - a prospective study and a retrospective study. The prospective study, is a case series study, using the snowball sampling method, starting with the first case reported. The general research method involves identifying index individuals and, along with collecting information on them, asking them to refer other persons suitable for the study [14]. Face-to-face interviews were carried out between May 10th and 23rd, 2022 with all possible cases identified and urine samples were collected for search S. haematobium. The retrospective study used a medical records review to search possible cases of SCHu.
Case definition: patient suspected with SCHu: a person who presented hematuria between September 1st, 2021, and May 10th, 2022 who went to the urgency service at Santa Rita Vieira Regional Hospital in the Santa Catarina municipality or in the Health Primary Care Center of Calheta in São Miguel municipality, in the North Santiago Sanitary Region or the Agostinho Neto University Hospital of Praia municipality. Confirmed case with SCHu: a suspected case for SCHu with a diagnostic positive for S. haematobium.
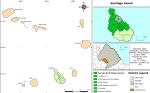
Study area: the studies were carried out in the North Santiago Sanitary Region which includes 6 municipalities: São Miguel; Santa Cruz, Santa Catarina; São Salvador do Mundo, São Lourenço dos Órgãos and Tarrafal. The first notified case resides in a rural region of the district of São Miguel called Cutelo Gomes (Figure 1).
Data collection: phone interviews were carried out between 29 May and 03 June 2022, and urine samples were collected from suspected cases. Three tentative calls were made to each suspect case. All interviews were made with the patients or a responsible person over 18 years of age when the patient was under 18 years of age. We used a questionnaire containing sociodemographic questions and illness exposures.
Data analysis methods: for data analysis, measures of relative, absolute, and central tendency were calculated, using Epi Info version 7.2. Tables and figures were made using Microsoft Excel 2013. Spatial characterization we are using QGis 2.18 software.
Laboratory methods
Collection of urine samples for parasite detection: urine samples were collected from all identified suspected. For urine collection, the patient jumped for 10 minutes before urinating. It is known that exercising before urinating increases the number of eggs in the urine [4]. The samples were collected in a sterile universal pot, kept refrigerated, and sent to the laboratory at the Regional Hospital with the request form for search for parasite eggs. The persons who were interviewed by telephone had urine collection scheduled for after the interview.
Laboratory diagnostic for S. haematobium: a sedimentation quantitative technique was employed for the detection of S. haematobium eggs in the urine samples as described by Cheesbrough [15]. Briefly, 10 ml of each urine sample was put in a centrifuge tube. The centrifuge was spun for 10 minutes at 3000 rpm. The sediment (after discarding the supernatant) was transferred to a clean dry glass slide covered with a cover slip and the sediment was examined microscopically using a 40x magnification to identify the terminal spine eggs of S. haematobium. The Regional Hospital laboratory performed the diagnosis, and the Central Hospital laboratory confirmed the diagnosis. The technique of cystoscopy with biopsy was also used for cases with persistent symptoms with negative for eggs in urine.
Environmental investigation
Collection, transport, and identification of snails: five field trips were carried out on different days to prospect for freshwater snails that are intermediate hosts of trematodes. The snails were collected using forceps and scoops in water bodies. They were also prospected in dry land where there were traces of water bodies. In the water bodies, the banks and the water column were inspected to capture snails. In dry places where water was present, soils were dug/scraped at a depth of approximately 10 cm for the prospection of snails. The back of plant leaves was also inspected in the surrounding vegetation in the water bodies. The transport of snails to the laboratory was in 100ml vials with a maximum of 15 specimens per vial. Species identification was performed by specimen observation under the Motic DM-143-FBGG optical stereomicroscope with a 5 times magnification objective, following the dichotomous key [16]. After 4 hours of exposure, the vial was observed under the stereomicroscope and kept separated from those that were not exposed.
Search of trematodes larval forms in snails: for the investigation of larval forms, cercariae, and miracidia in snails, two different techniques were applied: dissection of the snails and exposure to light and heat to verify the emission of cercariae. Dissection was carried out by placing the snails between the back of two glass petri dishes and applying light pressure until the shell cracks, without destroying the soft part of the animal. The shell pieces were removed with forceps and the snails´ soft bodies were observed under the stereomicroscope. Specimens were also dissected and mounted on slides and observed under an optical microscope with a 4-, 10- and 40-times objective, for the detection of larval forms of trematodes. The exposure of the snails to light was carried out with 3 or 4 specimens in 100 ml graduated Becker under a 40W incandescent lamp, at approximately 10cm, for at least 4 hours. The Beckers were placed on a hot plate to keep the temperature between 28-30°C, as the 40W lamp does not emit enough heat to increase the temperature of the water.
Ethics statement: health surveillance efforts aim for rapid disease containment through early detection, specific treatments, and elucidating distinctive elements of public health. The event was considered an outbreak of an emerging infectious disease. This way verbal consent was requested from suspected cases or parents who have the legal authority to give consent when under 18. The investigator explained to the interviewees that the individual and personal information would be confidential and protected, just after the verbal consent of the subjects, the interview continued. The published information is intended to enable effective measures in future outbreaks and support direct actions to improve the health of the population. In addition, disseminate it to the scientific community and international and national entities to generate public policies for this new disease described in the country.
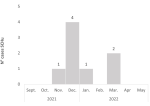
Prospective study: the first case was reported to the surveillance service of the municipality of São Miguel on May 5, 2022. It was a 7-year-old, male child, a student residing in Cutelo Gomes - a rural area of the city of São Miguel. From this case, it was possible to find 10 more patients. On the day of the interview, 7 (70%) had hematuria. Of the 11 interviewed cases, 7 were positive for SCHu, totalizing 8/11 (72.7%) with S. haematobium being 7 confirmed by S. haematobium eggs in urine sample and 1 by bladder biopsy. This last case was admitted at Central Hospital with progressive hematuria and was performed a cystoscopy with biopsies. Eggs of S. haematobium was demonstrated in one confirmed patient through the granulomatous inflammatory reaction observed around the eggs. Median age cases were 15 years (range: 7-24), all male and living in the area rural of São Miguel district, and the most frequent symptoms were hematuria, abdominal pain, and genital itching. Of the SCHu cases, 5 (65.5%) were students, and 3 (60%) of them studied at the same school. Three (37.5%) were hospitalized and searched more than two times for medical care before diagnosis and no deaths were registered (Table 1). The epidemic curve shows that the onset of symptoms in the index case was in November. It was only possible to obtain the exact date of onset of symptoms of three cases that had medical care (1st December 2021, 2nd December 2021 and 7 March 2022). Thus, the epidemic curve was constructed according to the month onset of symptoms (Figure 2). In the interviews of patients with SCHu, 4 (57.1%) had a habit of walking barefoot in water collections near the house 3 (75%) swam in water reservoir tanks and 3 (37.5%) reported seeing snails in the tanks or nearby. The water supply system in the region is carried out by auto tanks and the sewage system is a cesspool. Of the 10 interviewed cases, 8 (80%) do not perform any type of treatment for drinking water. When asked about the destination of the garbage collection, all deposit their garbage outdoors or burn the garbage outdoors (Table 2).
Retrospective study: between September 1st 2021, and May 10th 2022, 19,299 medical records were evaluated, from which 49 suspected SCHu cases were found. Twenty-three (23 (47%) were interviewed, 19 (82.6%) negatives for S. haematobium and 4 (17.4%) did not collect urine samples (Figure 3). Mean age 27 years (SD:16), 18 (78.3%) female, 3 (13%) had abdominal pain on the interview, 11 (47.8%) walking barefoot in water and 5 (26.1%) swam in water tanks. Of the 21 interviewed, 17 (81%) do not do any type of treatment for drinking water. When asked about the destination of the garbage collection, 12 (52.2%) have a garbage collection system and 11 (47.8%) burn the garbage outdoors. Sanitation information was not collected.
Environmental investigation: a total of 267 specimens of molluscs were collected, belonging to 4 different families (Figure 4).
The study revealed the existence of the disease in the country and raised environmental risk factors that increase the possibility of contracting schistosomiasis when in contact with infested waterbodies. In addition, the findings showed that school-age children were more affected. The attack rate among the confirmed cases interviewed showed that swimming or playing in water tanks is a plausible hypothesis for contamination by S. haematobium. The study revealed that walk barefoot in water collections near the house.
Other studies observed that children under the age of 15 years are more susceptible to infection due to certain play habits, such as fishing or swimming in infested water and lack of hygiene [17-19] Such factors include living close to water bodies and having rivers on the way to school. Furthermore, if left untreated or undiagnosed, by children below the age of 15 years, it can lead to a variety of long-term impacts such as anemia and iron deficiency, leading to school absenteeism, poor academic performance, and increased high-school dropout rates [18]. The ill has profound negative effects on child development, the outcome of pregnancy, and agricultural productivity, thus a key reason why the “bottom 500 million” inhabitants of sub-Saharan Africa continue to live in poverty [1].
Despite extensive environmental investigation, we did not find the intermediate host of S. haematobium in the area. However, in Cabo Verde, eight species of freshwater molluscs have been identified and six occur on Santiago Island [12,20]. It is believed that the disease host was not found because the water reservoirs and water bodies were empty due to the dry season. Previous malacological studies on temporary rain ecosystems in West Africa and in Central Africa have shown the importance of ponds, representing the habitats of the snail, the intermediate host of S. haematobium [21].
Another important finding that our study presents is related to the sanitary conditions with the lack of treatment of potable water and inadequate sanitation. Schistosomiasis can be controlled using key approaches adopted by the World Health Organization (WHO) which include adequate sanitation, potable water supply, effective treatment, and health education [3,18]. Health education programs on schistosomiasis must promote disease knowledge, especially for children, to reduce and control the disease.
There have been increased efforts to eliminate schistosomiasis in the past decade with WHO setting 2030 as the goal for transmission interruption in endemic African countries. Government agencies from many countries have prioritized the control of neglected tropical diseases by exploiting breakpoints in their lifecycles, such as the implementation of snail control and improvements in sanitation and access to safe, clean water. However, in sub-Saharan Africa, preventive chemotherapy is still the major intervention practiced [22]. Cabo Verde has the challenge of understanding the magnitude of the disease since there are no previous records. However, with many migrants from countries with a high prevalence, mainly from Guinea Bissau, and Senegal [17]. The national statistics in Senegal suggest that the prevalence of urogenital schistosomiasis ranges from 10% to more than 95% in the country [18]. Limitations of this study are possible recall bias caused by the delay between the illness and the investigation, with the first patient getting sick in November and the investigation starting in May. Also, the epidemic curve was not possible to be described by the date of onset of symptoms. The dry period may have probably prevented the finding of the transmitting snail reservoir.
This is the first human schistosomiasis description in Cabo Verde, and an outbreak of urinary schistosomiasis occurred in the rural area of Cutelo Gomes in São Miguel District. The description of the outbreak established an important record of the occurrence of Neglected tropical diseases (NTD) in a new territory. Thus, this scientific description establishes an important registry and message for national and international organizations and policymakers allowing the establishment of policies, strategies, and fund allocation to avoid the endemicity and expansion of the occurrence of this NTD in the country.
Recommendations, actions: we recommended that all SCHu cases be treated with praziquantel and those who presented clinically compatible with SCHu, but with a negative urine test for S. haematobium, should be to the Central Hospital for cystoscopy with biopsy. It is important to note that as a chronic disease, the persistent clinic of hematuria with an unknown cause should be investigated. The infection mechanism remains unknown because the specific reservoir genus of snails was not found (Bulinus). We recommended a survey and environmental assessment in the rainy season, which could elucidate risk factors for the acquisition of SCHu in this region. Information on the intensity and prevalence of schistosome infection among freshwater snails is scarce in many African countries, so policymakers need to give better attention to snail control strategies in Africa. In addition, is needed epidemiological inspections of adjacent schools and communities to better gauge the full footprint of urinary schistosomiasis with efforts to mitigate transmission with health education and appropriate interventions. Water quality is a real public health problem in the Cutelo Gomes region, especially for the population at risk. For them, alternative water supply or improved treatment should be implemented. An important risk factor for SCHu already described in the literature was fished or swim-infested water [18-20], so actions for education are necessary to decrease the of risk becoming ill, especially children and young people.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
We acknowledge the management of the Santa Rita Vieira Regional Hospital, a special acknowledgment to the laboratory’s professionals for the urine analysis of the patients identified in the outbreak. We acknowledge the medical team at the Agostinho Neto University Hospital the team of health service and the vector combat health agents of the São Miguel. We acknowledge too the director of the Sanitary Region of Santiago Norte - Dr. João Baptista Semedo for their support. We thank Dr. Silvania Leal of the Medical Entomology Laboratory of the National Institute of Public Health of Cabo Verde for supporting the structure of the laboratory for research on snails and Sr. Carlos and Sr. Allyson were drivers of the National Institute of Public Health of Cabo Verde of transport in the field.
Table 1: sociodemographic characteristics for positive patients with urinary schistosomiasis, São Miguel, Cabo Verde, 2024
Table 2: description of exposure to ill, type of water source, and the other public health problems of the interviewed in the case series prospective study, São Miguel, Cabo Verde, 2024
Figure 1: map of the North Santiago Sanitary Region, Santiago Island, Cabo Verde, 2024
Figure 2: curve epidemic according to the month onset of symptoms, São Miguel, Cabo Verde, 2024
Figure 3: flowchart retrospective search for cases of urinary schistosomiasis, Santiago North Region Sanitary, Cabo Verde, 2024
Figure 4: molluscs collected according to taxonomy by families, São Miguel, Cabo Verde, 2024: A) Planorbidae; B) Hydrobiidae; C) Lymnaeide; D) Thiaridae
- Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Brazilian Journal of Infectious Diseases. 2015;19(2):196-205. PubMed | Google Scholar
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. The Lancet Infectious Diseases. 2006;6(7):411-425. PubMed | Google Scholar
- WHO. Schistosomiasis Fact sheet. 2022. Accessed on Aug 13, 2023.
- Chandler AC, Read CP. Introduction to Parasitology with special reference to the Parasites of Man. Introduction to Parasitology with special reference to the Parasites of Man. 1961(Edn 10). Google Scholar
- Zhou X, Bergquist R, Leonardo LR, Olveda R. Schistosomiasis: the disease and its control. Regional network for research. Surveillance and: Control for Asian Schistosomiasis. 2008. Google Scholar
- Ajakaye OG, Adedeji OI, Ajayi PO. Modeling the risk of transmission of schistosomiasis in Akure North Local Government Area of Ondo State, Nigeria using satellite derived environmental data. LoS Negl Trop Dis. 2017 Jul 12;11(7):e0005733. PubMed | Google Scholar
- Heyns CF. Urinary Tract Diseases. Hunter´s Tropical Medicine and Emerging Infectious Disease: Ninth Edition. 2013;47-53.
- Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. The Lancet. 2014;383(9936):2253-2264. PubMed | Google Scholar
- Chahdi H, Damiri A, El Ochi MR, Allaoui M, Al Bouzidi A, Oukabli M. Urinary schistosomiasis: report of case diagnose in bladder biopsy. BMC Clin Pathol. 2018 Nov 28:18:13. PubMed | Google Scholar
- Geleta S, Alemu A, Getie S, Mekonnen Z, Erko B. Prevalence of urinary schistosomiasis and associated risk factors among Abobo Primary School children in Gambella Regional State, southwestern Ethiopia: A cross sectional study. Parasit Vectors. 2015 Apr 10:8:215. PubMed | Google Scholar
- Zhang SM, Bu L, Lu L, Babbitt C, Adema CM, Loker ES. Comparative mitogenomics of freshwater snails of the genus Bulinus, obligatory vectors of Schistosoma haematobium, causative agent of human urogenital schistosomiasis. Sci Rep. 2022 Mar 30;12(1):5357. PubMed | Google Scholar
- Crespo MV, Rosa F, Évora C. Interactions between the environment and parasites on Santiago Island (Cape Verde) (Interações entre o meio ambiente e parasitas na Ilha de Santiago. In: I Congresso de desenvolvimento Regional de Cabo Verde/ II Congressso Lufosófono de Ciência Regional). 2001:278-296. Google Scholar
- Crespo MV, Rosa F. Interactions with the environment, domestic animals, parasites on Santiago Island. In: Proceedings of the International Conference Cape Verde and Guinea-Bissau: Paths of Knowledge and Science (Interações ambiente, animais domésticos, parasitas na Ilha de Santiago. In: Atas do Coloquio Internacional Cabo Verde e Guiné-Bissau: Percursos do Saber e da Ciência). 2012: 21-23.
- Kennedy-Shaffer L, Qiu X, Hanage WP. Snowball Sampling Study Design for Serosurveys Early in Disease Outbreaks. Am J Epidemiol. 2021 Sep 1;190(9):1918-1927. PubMed | Google Scholar
- Cheesbrough M. District Laboratory Practice in Tropical Countries. Cambridge University Press 2nd edition. 2005. Google Scholar
- Mandahl-Barth G. Key to the identification of east and central African freshwater snails of medical and veterinary importance. Bull World Health Organ. 1962;27(1):135-50. PubMed | Google Scholar
- Frigerio S, Bert F, Clari M, Fine GD, Riva S, Bergese I et al. Knowledge, Attitudes, and Practices Related to Schistosomiasis Among Children in Northern Senegal. Annals of Global Health. 2016;82(5):840-847. PubMed | Google Scholar
- Hambury SD, Grobler AD, Melariri PE. Knowledge, attitudes, and practices on urinary schistosomiasis among primary schoolchildren in Nelson Mandela Bay, South Africa. J Parasitol Res. 2021 Nov 2:2021:6774434. PubMed | Google Scholar
- Wiegand RE, Fleming FM, Straily A, Montgomery SP, Vlas SJ de, Utzinger J et al. Urogenital schistosomiasis infection prevalence targets to determine elimination as a public health problem based on microhematuria prevalence in school-age children. PLoS Negl Trop Dis. 2021 Jun 11;15(6):e0009451. PubMed | Google Scholar
- Costa FL, Crespo MV, Correia E. Evolution of trematode/mollusc aquatic biotopes (Evolução dos Biótopos Aquáticos de Trematódeos - Moluscos em Cabo Verde. In: Costa FL, F R, V C, editors. 1 Conferência Lusofona sobre Sistemas Terra). 2006.
- Senghor B, Diaw OT, Doucoure S, Seye M, Talla I, Diallo A et al. Study of the snail intermediate hosts of urogenital schistosomiasis in Niakhar, region of Fatick, West central Senegal. Parasit Vectors. 2015 Aug 7;8:410.
- Aula OP, McManus DP, Jones MK, Gordon CA. Schistosomiasis with a focus on Africa. Tropical Medicine and Infectious Disease. 2021;6(3). PubMed | Google Scholar
Search
This article authors
On Pubmed
- Veruska Maia da Costa
- Menilita Paula Varela dos Santos Barbosa
- Isaias Baptista Fernandes Varela
- Aniceto Tavares dos Santos
- Jonas Antônio Lopes Gomes
- Graça Maria Carvalho Mendes Moniz
- Ludmila dos Santos Miranda
- Jaelsa Mira Gonçalves Moreira
- Liliane Margareth Teixeira Hungria Silva
- Jonas Lotufo Brant de Carvalho
- Maria da Luz Lima Mendonça
On Google Scholar
- Veruska Maia da Costa
- Menilita Paula Varela dos Santos Barbosa
- Isaias Baptista Fernandes Varela
- Aniceto Tavares dos Santos
- Jonas Antônio Lopes Gomes
- Graça Maria Carvalho Mendes Moniz
- Ludmila dos Santos Miranda
- Jaelsa Mira Gonçalves Moreira
- Liliane Margareth Teixeira Hungria Silva
- Jonas Lotufo Brant de Carvalho
- Maria da Luz Lima Mendonça