Assessment of COVID-19 vaccine hesitancy among people living with HIV/AIDS: a single-centered study
Muktar Musa Shallangwa, Shuaibu Saidu Musa, Honesty Chukwudi Iwenya, Emery Manirambona, Don Eliseo Lucero-Prisno III, Babayo Muhammad Tukur
Corresponding author: Shuaibu Saidu Musa, Department of Nursing Science, Ahmadu Bello University, Zaria, Nigeria 
Received: 22 Oct 2022 - Accepted: 03 Jan 2023 - Published: 12 Jan 2023
Domain: Biostatistics,Environmental health,HIV epidemiology
Keywords: COVID-19, vaccine, hesitancy, HIV/AIDS, State Specialist Hospital Maiduguri, Nigeria
©Muktar Musa Shallangwa et al. PAMJ-One Health (ISSN: 2707-2800). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Muktar Musa Shallangwa et al. Assessment of COVID-19 vaccine hesitancy among people living with HIV/AIDS: a single-centered study. PAMJ-One Health. 2023;10:2. [doi: 10.11604/pamj-oh.2023.10.2.37945]
Available online at: https://www.one-health.panafrican-med-journal.com/content/article/10/2/full
Research 
Assessment of COVID-19 vaccine hesitancy among people living with HIV/AIDS: a single-centered study
Assessment of COVID-19 vaccine hesitancy among people living with HIV/AIDS: a single-centered study
![]() Muktar Musa Shallangwa1, Shuaibu Saidu Musa2,3,&,
Muktar Musa Shallangwa1, Shuaibu Saidu Musa2,3,&, ![]() Honesty Chukwudi Iwenya4,
Honesty Chukwudi Iwenya4, ![]() Emery Manirambona5, Don Eliseo Lucero-Prisno III6,7,8, Babayo Muhammad Tukur9
Emery Manirambona5, Don Eliseo Lucero-Prisno III6,7,8, Babayo Muhammad Tukur9
&Corresponding author
Introduction: as the coronavirus disease 2019 (COVID-19) vaccines are distributed and administered globally, hesitancy towards the vaccine hinders the immunisation of a significant number of vulnerable populations, such as people living with HIV/AIDS. Hence, this study aims to assess COVID-19 vaccine hesitancy among people living with HIV/AIDS (PLHIV) attending clinical-outpatient follow-up at State Specialist Hospital Maiduguri (SSHM), Borno State, Nigeria.
Methods: a hospital-based cross-sectional study design was conducted to assess COVID-19 vaccine hesitancy among 344 PLHIV receiving antiretroviral therapy (ART) at the United States President´s Emergency Plan for AIDS (PEPFAR) clinic in SSHM from 4th January to 25th February 2022. Data were collected using a structured and pretested interviewer-administered questionnaire. The results were presented using frequencies and percentages. The factors that are associated with COVID-19 vaccine hesitancy were identified using the Chi-square statistical test.
Results: among the 344 respondents of the study, only 88 (26.6%) received the COVID-19 vaccine. Out of the 256 respondents that did not receive the vaccine, only 10.5% (27/256) are willing to be vaccinated, while the majority; 57.8% (148/256) are not willing to be vaccinated and 31.7% (81/256) of the respondents are uncertain, thus resulting in a hesitation rate of 89.45%. There was no statistically significant association between COVID-19 vaccine hesitancy and the study´s independent variables; where p-value is greater than 0.05.
Conclusion: hesitancy towards COVID-19 vaccine is high among PLHIV and there is no any statistically significant association between COVID-19 vaccine hesitancy and the independent variables of the study where p-value is greater than 0.05. Hence, it is necessary to develop targeted strategies to boost vaccine uptake among this vulnerable population.
The coronavirus disease 2019 (COVID-19) outbreak is a socio-economic and global health threat, which attempts are being made to prevent and manage its spread [1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly worldwide as the primary agent of the COVID-19 pandemic since its debut in late 2019 [2]. The clinical course of COVID-19 has shown asymptomatic illness in some patients at the initial stages of the disease, other patients developed cough, mild fever, muscle pain, joint pain, and chest pain, shortness of breath, runny nose and general body weakness [3]. Key outcomes in the later stages were severe acute respiratory distress and mortality in the Intensive Care Unit (ICU) due to organ malfunction, bleeding, and septic shock [4]. The upsurge of the COVID-19 variants such as the Delta and the Omicron threatens the efforts to curb the spread of the virus, especially in African countries, where; Nigeria and Ghana were the first countries to report cases of Omicron variant in West Africa. Increased Omicron variant instances in Botswana, South Africa, and several West African nations, such as Nigeria and Ghana, might spell disaster for the continent's health system and further devastate the continent's faltering economies [5].
Since the majority of asymptomatic and mild infections of COVID-19 go unrecorded, published figures are likely to be underestimated [6]. However, as of 28th October 2022, there have been 626,337,158 confirmed cases of COVID-19 infection with 6,566,610 deaths worldwide [7]. Nigeria recorded a total of 266,043 confirmed cases and 3,155 deaths as of 30th October 2022 [8]. COVID-19 related outcomes are worse in vulnerable populations, such as people living with HIV/AIDS (PLHIV) than in the general population [9-11]. Despite the significant progress in expanding HIV/AIDS treatment coverage in recent decades, the high incidence of HIV/AIDS has remained a key problem globally [12]. The United Nations Agency for International Development (UNAIDS) 2021 Global HIV/AIDS statistics factsheet unveiled that, the incidence of HIV infection was 1.5 million in 2020 and a total of 37.7 million people were living with HIV, with a total of 36.3 million death cases from AIDS-related illness since the inception of the epidemic. Reports have shown that the COVID-19 pandemic has impacted HIV prevention services, HIV testing, HIV treatment, and viral load suppression, perhaps, resulting in a loss of control over the HIV epidemic [13]. Although there is little evidence on how HIV infection influences the chance of poor COVID-19 results [14], according to a report from the World Health Organization, HIV infection significantly increases the probability of both severe/critical COVID-19 presentation at hospital admission and in-hospital death [15]. Previous studies in Spain and the United Kingdom show that, HIV-positive patients had greater rate of serious diseases [16] and risk of COVID-19 death [17] than HIV-negative people. The higher vulnerability expressed by PLHIV to COVID-19 may be probably due to their impaired immune system [18].
In the absence of a universal treatment option for COVID-19, the most reliable and cost-effective strategy to prevent COVID-19 and control its spread is the COVID-19 vaccination [19]. To mitigate the virus's negative impact on public health and the global economy, significant progress has been achieved in developing effective and safe COVID-19 vaccines [20,21] where 12,830,378,906 vaccine doses were administered globally as of 26th October 2023 [7]. The Nigerian government obtained numerous vaccination lots through the COVID-19 Vaccines Global Access Facility (COVAX), a collaboration of the Coalition for Epidemic Preparedness Innovations (CEPI), Gavi the Vaccine Alliance, United Nations Children's Fund (UNICEF), World Bank, and the World Health Organization [22]. Thereafter, Nigeria's National Primary Health Care Development Agency (NPHCDA) began vaccinating priority groups in stages, beginning with frontline healthcare professionals. Despite the paucity of information on COVID-19 vaccine safety in PLHIV, the WHO recommended that COVID-19 vaccines are safe for PLHIV [23] although, the effectiveness of the vaccine is totally dependent on its uptake [24].
Herd immunity is frequently pursued to bring a disease outbreak under control in vaccination [25]. Herd immunity against COVID-19 infection is expected to be reached when at least 70% of the population is immune, either by vaccination or natural infection [26,27] and Nigeria has only 20.9% of its population fully vaccinated against the COVID-19 [28]. As a result, achieving high vaccine coverage rates is one of the most effective ways for reducing COVID-19-related morbidity and mortality [29]. As COVID-19 vaccines are disseminated and administered in many countries, including Nigeria, vaccination hesitancy is becoming a challenge and a barrier to reaching a large proportion of the susceptible population [1] such as PLHIV. The WHO's Strategic Advisory Group of Experts on Immunization (SAGE) defined vaccine hesitancy as the delay in accepting or refusing immunization notwithstanding the availability of vaccination services [30]. COVID-19 vaccine hesitancy has been seen as a worldwide issue. This poses a serious barrier to global efforts to contain the COVID-19 pandemic [24] further, exposing the vulnerable populations such as PLHIV to a greater risk. Hesitation to get vaccinated is complicated and context dependent, ranging over time, region, and type of vaccine, and impacted by characteristics including complacency, comfort, and confidence [31]. Hence, this study aims to assess COVID-19 vaccine hesitancy among PLHIV on ART at SSHM. We anticipated that the findings from this study will provide crucial information that will guide relevant authorities in improving COVID-19 vaccine uptake among PLHIV.
Study design: a hospital-based cross-sectional study design was conducted to assess COVID-19 vaccine hesitancy among PLHIV receiving ART at the United States President´s Emergency Plan for AIDS Relief (PEPFAR) clinic in State Specialist Hospital Maiduguri (SSHM).
Study setting and population: State Specialist Hospital Maiduguri (SSHM) is one of the government-owned secondary healthcare facilities within Maiduguri metropolis, Borno State. The hospital had a total of 3309 PLHIV currently on ART at the time of the study. Respondents that participated in the study are PLHIV receiving ART at SSHM. Inclusion criteria for the study includes being aware of their HIV status; being on ART at SSHM; and being at least 15 years old. The respondents of this study were recruited from an earlier study that assessed the knowledge attitude and practice towards COVID-19 among PLHIV in the same setting [32].
Data resource and measurement
Data collection tool: a structured interviewer-administered questionnaire prepared in English language based on relevant published articles [33,34] and was translated to the participants in their respective local languages for proper understanding and responses.
Data collection: data were collected by three research assistants from 4th January to 25th February 2022 at SSHM. The questionnaire consists of two different sections. The first section contained information on the participants´ socio-demographic characteristics, including sex, age, marital status, educational status, employment status and duration on ART. In the second section, participants were asked whether they have received COVID-19 vaccine or if they would be willing to receive the vaccine. Possible reasons for COVID-19 vaccine hesitancy were also assessed. The response modalities were "yes" or "no". A pretest using the prepared questionnaire was carried out among 20 PLHIV who were chosen at random at SSHM and did not participate in the main study in order to determine the questionnaire´s reliability. The pretested questionnaire was then administered to the research participants.
Sample size: Cochran´s formula [35] was used to calculate the sample size at 95% level of confidence where Z is taken at 1.96.
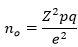
Due to the lack of a comparable study in the study's location, the prevalence was estimated to be 50%, and the margin of error (e) was set at 5%. The sample size was calculated using the formula to be 384. Cochran's correction formula was used to further adjust the sample size since the study's target population is less than 10,000.
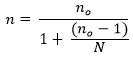
Where n is the corrected sample size, no is the uncorrected sample size and N is the population size. This correction formula yielded a final sample size of 344. After then, respondents were selected using convenience sampling.
Data analysis: data were checked for completeness and consistency, coded, and entered to Microsoft Excel, 2016 and statistical analysis of the data was performed with Statistical Package for Social Sciences (Version 26). Characteristics of the study participants were described in terms of frequencies and percentages for all the variables. The Chi-square (X2) statistical test was used to measure the association between the dependent variables (sex, age, marital status, educational status, employment status and duration on ART) and independent variable (COVID-19 vaccine hesitancy) at the bivariate level where; P-values were considered significant at values greater 0.05.
Ethical consideration: the State Specialist Hospital Maiduguri's ethical review and clearance committee provided a letter of approval for the study to be conducted. Study participants' anonymity was maintained, and no personal identifiers were used on the data collection forms. The aim and objectives of the research project were explained to the respondents, and they were assured that their treatment and other services they gain from the hospital will not be influenced by their participation in the study. Finally, they were asked for their informed consent for participation and use of their records for the study and its publication.
Socio-demographic analysis: the socio-demographic characteristics of the respondents are shown in Table 1. Majority, 70.3% (242/344) of the respondents were females and most of the participants, 58.7% (202/344) are 25 years and above. 50.6% (174/344) of the respondents were married, 63.4% (218/344) were literate and only 20.3% (70/344) were employed. All the respondents were receiving ART at Maiduguri State Specialist Hospital (MSSH) and most of them, 56.4% (194/344) were diagnosed with HIV for 5 years or more.
COVID-19 vaccine uptake analysis: of the 344 respondents, only 88 have received COVID-19 vaccine, yielding an uptake rate of 25.6%. Out of the 256 respondents that have not received the vaccine, only 10.5% (27/256) are willing to receive the vaccine while majority, 57.8% (148/256) are not willing to receive the vaccine, and 31.7% (81/256) of the respondents are undecided, thus yielding a hesitancy rate of 89.45% as shown in Table 2.
Reasons for COVID-19 vaccine hesitancy analysis: among the 229 respondents that were hesitant about the COVID-19 vaccine uptake, majority, 46.3% (106/229) cited being worried about vaccine side effects as their reason for the hesitations. Nineteen point two percent (19.2% (44/229)) had little or no confidence that the vaccine can protect against COVID-19 and 10% (23/229) reported not knowing anyone who died of COVID-19 as their reason for hesitancy. Fourteen percent (14% (32/229)) of the respondents needed more information on the COVID-19 vaccine than what is currently being given to the public while; 10.5% (24/229) did not believe that COVID-19 is real in Nigeria as shown in Table 3.
Bivariate analysis of factors associated with COVID-19 vaccine hesitancy: Table 4 shows the association between the independent variables of the study and COVID-19 vaccine hesitancy of the respondents. There is no any statistically significant association between COVID-19 vaccine hesitancy and the independent variables of the study; where P-value is greater than 0.05.
Hesitancy may impair COVID-19 vaccine uptake and hinder the establishment of herd immunity as COVID-19 vaccines are developed and gradually made available [36]. Our study is aimed at assessing COVID-19 vaccine hesitancy among PLHIV; among whom the majority were women, aged 25 years and above, illiterates and unemployed. Despite PLHIV have an increased risk of COVID-19 associated morbidity and mortality [37], our findings revealed that only 25.6% of the participants received the COVID-19 vaccine. This percentage is lower than that of a similar study conducted in USA, where the COVID-19 vaccine uptake rate was reported to be 64% among PLHIV [38]. This low uptake rate reported is largely due to vaccine hesitancy. Our findings also show that only 10.5% of the participants that are yet to receive the COVID-19 vaccine are willing to be vaccinated while, 89.45% are unwilling or hesitant about getting vaccinated. This finding is congruent with that of a similar study conducted in Kano city of northern Nigeria, where COVID-19 vaccine acceptance was reported to be low among PLHIV [39]. The COVID-19 vaccine hesitancy rate reported in this study is higher than in previous studies among PLHIV [19,35,40]. The high rate of the COVID-19 vaccine hesitancy may largely be attributed to COVID-19 safety concerns, where 46.3% of the participants that are hesitant towards the vaccine cited being worried about the vaccine side effects, despite the WHO, Nigeria's drug regulatory agency, and the National Agency for Food and Drug Administration and Control, approving the COVID-19 vaccine [41]. The safety concerns may be linked to the accelerated development of the vaccine where some people believed that the safety and efficacy of the vaccines may not have been completely captured [42]. Other factor that might have fueled the safety concern is misinformation from social media by anti-COVID-19 vaccine groups. Therefore, the federal and state ministries of health (MOH) should intensify their COVID-19 vaccine public enlightenment campaigns so as to address this concern in order to persuade more people especially the vulnerable groups such as PLHIV to get vaccinated and mitigate corona virus infection.
Other reasons for COVID-19 vaccine hesitancy that was revealed in this study are: lack of total confidence on the vaccine, disbelieve that people can die as a result of COVID-19 infection, inadequate public awareness on the COVID-19 vaccine and disbelieve in the existence of COVID-19 in Nigeria as 10.5% of the participants who are hesitant towards the vaccine do not believe that COVID-19 is real in Nigeria despite the high number of confirmed cases reported in the country. This is mainly due to lack of trust in government, where some people view COVID-19 reports in Nigeria as a hoax and a means of siphoning public funds by corrupt government officials. There was no statistically significant association between the independent variables of the study and vaccine hesitancy, with P-values greater than 0.05. This result is dissimilar to previous studies that reported age, sex, educational status, marital status and duration on ART and diagnosis being associated with vaccine hesitancy [1,22,38,43,44]. The discrepancy might be due to the low sample size used in this study. Therefore, future studies about COVID-19 hesitancy among PLHIV should use a larger sample size. Our study had a few limitations. Results from the study cannot be generalized because it was a single-centred study with a small sample size. Moreover, the proportion reported to have been vaccinated may be an overestimation of the actual figure. This may have been avoided with a more objective approach to evaluation. Furthermore, unmeasured confounding factors such as prior vaccination experiences could have influenced vaccine hesitancy on a personal level. Despite these drawbacks, the study greatly adds to our understanding of COVID-19 vaccine hesitancy, particularly among PLHIV.
The study assessed COVID-19 vaccine hesitancy among PLHIV. Our findings show that COVID-19 vaccine hesitancy is high among PLHIV mainly due to safety concerns associated with the use of the vaccine. There was no statistically significant association between the independent variables of the study and vaccine hesitancy, where; P-values were significantly considered at values greater than 0.05. As a result, it is recommended that decision-makers at the state and federal ministries of health and other concerned parties such as civil society organisations and non-governmental organisations engaged in HIV care intensify their COVID-19 vaccination sensitisation programs, focusing primarily on health education campaigns about the vaccine's safety and efficacy. Antiretroviral therapy (ART) centres should be leveraged as media for providing accurate information and addressing misconceptions about the vaccine among PLHIV as they receive care and support services because they are more likely to believe vaccine-related information supplied by their primary care providers. Hence, COVID-19 vaccine sensitisation campaigns should also be improved at ART centres.
What is known about this topic
- PLHIV have an increased risk of COVID-19 associated morbidity and mortality;
- Age, sex, educational status, marital status and time since HIV diagnosis are associated with vaccine hesitancy.
What this study adds
- COVID-19 vaccine hesitancy is high among PLHIV in Maiduguri, mainly due to safety concerns associated with the use of the vaccine;
- Other reasons for COVID-19 vaccine hesitancy that was revealed in this study are: lack of total confidence on the vaccine, disbelieve that people can die as a result of COVID-19 infection, inadequate public awareness on the COVID-19 vaccine and disbelieve in the existence of COVID-19 in Nigeria;
- Our findings also show that out of the participants, only few (10.5%) that are yet to receive the COVID-19 vaccine are willing to be vaccinated.
The authors declare no competing interest.
All authors contributed equally, read and approved the final manuscript for publication.
The authors would like to thank the SSHM PEPFAR clinic staff for their support, as well as the research assistants who helped with data collection and the PLHIV without whose consent; the study would not have been conducted.
Table 1: sociodemographic characteristics of PLHIV at SSHM, Borno State, Nigeria, 2022
Table 2: COVID-19 vaccine uptake analysis among PLHIV at SSHM, Borno State, Nigeria, 2022
Table 3: reasons for COVID-19 vaccine hesitancy analysis among PLHIV at SSHM, Borno State, Nigeria, 2022
Table 4: bivariate analysis of factors associated with COVID-19 vaccine hesitancy among PLHIV at SSHM Borno State, Nigeria, 2022
- Mohammed R, Nguse TM, Habte BM, Fentie AM, Gebretekle GB. COVID-19 vaccine hesitancy among Ethiopian healthcare workers. PLoS ONE. 2021;16(12):e0261125. PubMed | Google Scholar
- Helmy YA, Fawzy M, Elaswad A, Sobieh A, Kenney SP, Shehata AA. The COVID-19 Pandemic: A Comprehensive Review of Taxonomy, Genetics, Epidemiology, Diagnosis, Treatment, and Control. Journal of Clinical Medicine. 2020;9(4):1225. PubMed | Google Scholar
- Ji HL, Zhao R, Matalon S., Matthay MA. Elevated Plasmin(ogen) as a Common Risk Factor for COVID-19 Susceptibility. Physiological Review. 2020;100(3):1065-75. PubMed | Google Scholar
- Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422. PubMed | Google Scholar
- Musa SS, Gyeltshen D, Manirambona E, Ayuba D, Lucero-Prisno DE 3rd. The new COVID-19 Omicron variant: Africa must watch its spread. Clinal Epidemiology Global Health. 2022;13:100961. PubMed | Google Scholar
- Choi EM. COVID-19 vaccines for low- and middle-income countries. Trans RSoc Tropical Medicine & Hygiene. 2021 May 8;115(5):447-456. PubMed | Google Scholar
- WHO. WHO Coronavirus (COVID-19) Dashboard. Accessed October 30, 2022.
- NCDC. COVID-19 Nigeria. Accessed October 30, 2022.
- Bogart LM, Ojikutu BO, Tyagi K, Klein DJ, Mutchler MG, Dong L et al. COVID-19 Related Medical Mistrust, Health Impacts, and Potential Vaccine Hesitancy Among Black Americans Living With HIV. Journal of Acquired Immune Deficiency Syndrome. 2021;86(2):200-207. PubMed | Google Scholar
- Tesoriero JM, Swain CAE, Pierce JL, Zamboni L, Wu M, Holtgrave DR et al. COVID-19 Outcomes Among Persons Living with or Without Diagnosed HIV Infection in New York State. JAMA Netw Open. 2021;4(2):e2037069. PubMed | Google Scholar
- Waterfield KC, Shah GH, Etheredge GD, Ikhile O. Consequences of COVID-19 crisis for persons with HIV: the impact of social determinants of health. BMC Public Health. 2021;21(1):299. PubMed | Google Scholar
- UNAIDS. Global HIV & AIDS statistics-2021 fact sheet. Geneva; 2021. Accessed April 10, 2022.
- Brown LB, Spinelli MA, Gandhi M. The interplay between HIV and COVID-19: summary of the data and responses to date. Current. Opinion in HIV AIDS. 2021;16(1):63-73. PubMed | Google Scholar
- Fung M, Babik JM. COVID-19 in Immunocompromised Hosts: What We Know So Far. 2021;72(2):340-350. PubMed | Google Scholar
- Bertagnolio S, Thwin SS, Silva R, Ford N, Baggaley R, Vitoria M et al. Clinical features and prognostic factors of COVID-19 in people living with HIV hospitalized with suspected or confirmed SARS-CoV-2 infection. 11th IAS Conference on HIV Science, July. 2021;abstract PEBLB20. Google Scholar
- Vizcarra P, Pérez-Elías MJ, Quereda C, Moreno A, Vivancos MJ, Dronda F et al. Description of COVID-19 in HIV-infected individuals: a single-centre, prospective cohort. 2021;7(8):e554-e564. PubMed | Google Scholar
- Bhaskaran K, Rentsch CT, MacKenna B, Schultze A, Mehrkar A, Bates CJ et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2020;8(1):e24-e32. PubMed | Google Scholar
- Varshney K, Ghosh P, Iriowen R. Risk Factors for COVID-19 Mortality Among People Living with HIV: A Scoping Review. AIDS Behav. 2022;26(7):2256-2265. PubMed | Google Scholar
- Liu Y, Han J, Li X, Chen D, Zhao X, Qiu Y et al. COVID-19 Vaccination in People Living with HIV (PLWH) in China: A Cross Sectional Study of Vaccine Hesitancy, Safety, and Immunogenicity. Vaccines. 2021;9(12):1-14. PubMed | Google Scholar
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med. 2020;383(20):1920-1931. Google Scholar
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516-527. PubMed | Google Scholar
- Mustapha M, Lawal BK, Sha´aban A, Jatau AI, Wada AS, Bala AA et al. Factors associated with acceptance of COVID-19 vaccine among University health sciences students in Northwest Nigeria. PLoS ONE. 2021;16(11):1-15. PubMed | Google Scholar
- Huang X, Yu M, Fu G, Lan G, Li L, Yang J et al. Willingness to Receive COVID-19 Vaccination Among People Living With HIV and AIDS in China: Nationwide Cross-sectional Online Survey. JMIR Public Health and Surveillance. 2021;7(10):1-18. PubMed | Google Scholar
- Rodriguez VJ, Alcaide ML, Salazar AS, Montgomerie EK, Maddalon MJ, Jones DL. Psychometric Properties of a Vaccine Hesitancy Scale Adapted for COVID-19 Vaccination Among People with HIV. AIDS and Behavior. 2022;26(1):96-101. PubMed | Google Scholar
- Uzochukwu IC, Eleje GU, Nwankwo CH, Chukwuma GO, Uzuke CA, Uzochukwu CE et al. COVID-19 vaccine hesitancy among staff and students in a Nigerian tertiary educational institution. Ther Adv Infect Dis. 2021 Nov 1;8:20499361211054923. PubMed | Google Scholar
- Anderson RM, Vegvari C, Truscott J, Collyer BS. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet. 2020;396(10263):1614-1616. PubMed | Google Scholar
- WHO. Immunization December 2019. Accessed March 28, 2022.
- Our World in Data. Coronavirus (COVID-19) Vaccinations. Accessed October 30, 2022.
- Jones DL, Salazar AS, Rodriguez VJ, Balise RR, Starita CU, Morgan K et al. Severe Acute Respiratory Syndrome Coronavirus 2: Vaccine Hesitancy Among Underrepresented Racial and Ethnic Groups With HIV in Miami, Florida. Open Forum Infect Dis. 2021 Mar 26;8(6):ofab15. PubMed | Google Scholar
- Wagner AL, Masters NB, Domek GJ, Mathew JL, Sun X, Asturias EJ et al. Comparisons of Vaccine Hesitancy across Five Low- and Middle-Income Countries. Vaccines. 2019;7(14):155. PubMed | Google Scholar
- Omer SB, Salmon DA, Orenstein WA, deHart MP, Halsey N. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. New England Journal of Medicine. 2009;360(19):1981-8. PubMed | Google Scholar
- Shallangwa MM, Iwenya H, Musa SS, Manirambona E, Hameed MA, Lucero-Prisno III DE. Knowledge, attitude and practice towards COVID-19 among people living with HIV/AIDS attending State Specialist Hospital Maiduguri, Borno State, Nigeria. PAMJ - One Health. 2022;8:10. Google Scholar
- Adigwe OP. COVID-19 vaccine hesitancy and willingness to pay: Emergent factors from a cross-sectional study in Nigeria. Vaccine X. 2021;9:100112. PubMed | Google Scholar
- Ekstrand ML, Heylen E, Gandhi M, Steward WT, Pereira M, Srinivasan K. COVID-19 Vaccine Hesitancy Among PLWH in South India: Implications for Vaccination Campaigns. Journal of Acquired Immune Deficiency Syndromes. 2021;88(5):421-425. PubMed | Google Scholar
- Cochran WG. Sampling techniques (3rd ed.). New York: John Wiley & Sons. 1977.
- Craxì L, Casuccio A, Amodio E, Restivo V. Who should get COVID-19 vaccine first? A survey to evaluate hospital workers´ opinion. Vaccines (Babel). 2021;9(3):189. PubMed | Google Scholar
- Garg S, Kim L, Whitaker M, O´Halloran A, Cummings C, Holstein R et al. Hospitalization rates and characteristics of patients hospitalized with laboratory confirmed coronavirus disease 2019. COVID-NET, 14 states, March 1-30, 2020. Morbidity and Mortality Weekly Report. 2020;69(15):458-464. PubMed | Google Scholar
- Jaiswal J, Krause KD, Martino RJ, D´Avanzo PA, Griffin M, Stults CB et al. SARS-CoV-2 Vaccination Hesitancy and Behaviors in a National Sample of People Living with HIV. AIDS Patient Care and STDs. 2021;36(1):34-44. PubMed | Google Scholar
- Vallée A, Fourn E, Majerholc C, Touche P, Zucman D. COVID-19 vaccine hesitancy among french people living with HIV. Vaccines. 2021;9(4):1-9. PubMed | Google Scholar
- Iliyasu Z, Kwaku AA, Umar A, Amina T, Ahmed F, Nass S et al. Predictors of COVID-19 Vaccine Acceptability among Patients Living with HIV in Northern Nigeria: A Mixed Methods Study. Curr HIV Res. 2022;20(1):82-90. PubMed | Google Scholar
- WHO. Status of COVID-19 vaccines with WHO-EUL/PQ evaluation process. . Accessed April 10, 2022.
- Fadda M, Albanese E, Suggs LS. When a COVID-19 vaccine is ready, will we all be ready for it. International Journal of Public Health. 2020;65(6):711-712. PubMed | Google Scholar
- Wu J, Li Q, Silver TC, Wang M, Gu J, Wei W et al. COVID-19 Vaccine Hesitancy Among Chinese Population: A Large-Scale National Study. Frontiers in Immunology. 2021;12:781161. PubMed | Google Scholar
- Amuzie CI, Odini F, Kalu KU, Izuka M, Nwamoh U, Emma-Ukaegbu U et al. COVID-19 vaccine hesitancy among healthcare workers and its socio-demographic determinants in Abia state, Southeastern Nigeria: A cross-sectional study. Pan Afr Med J. Pan Afr Med J. 2021 Sep 3;40:10. PubMed | Google Scholar




